Abstract
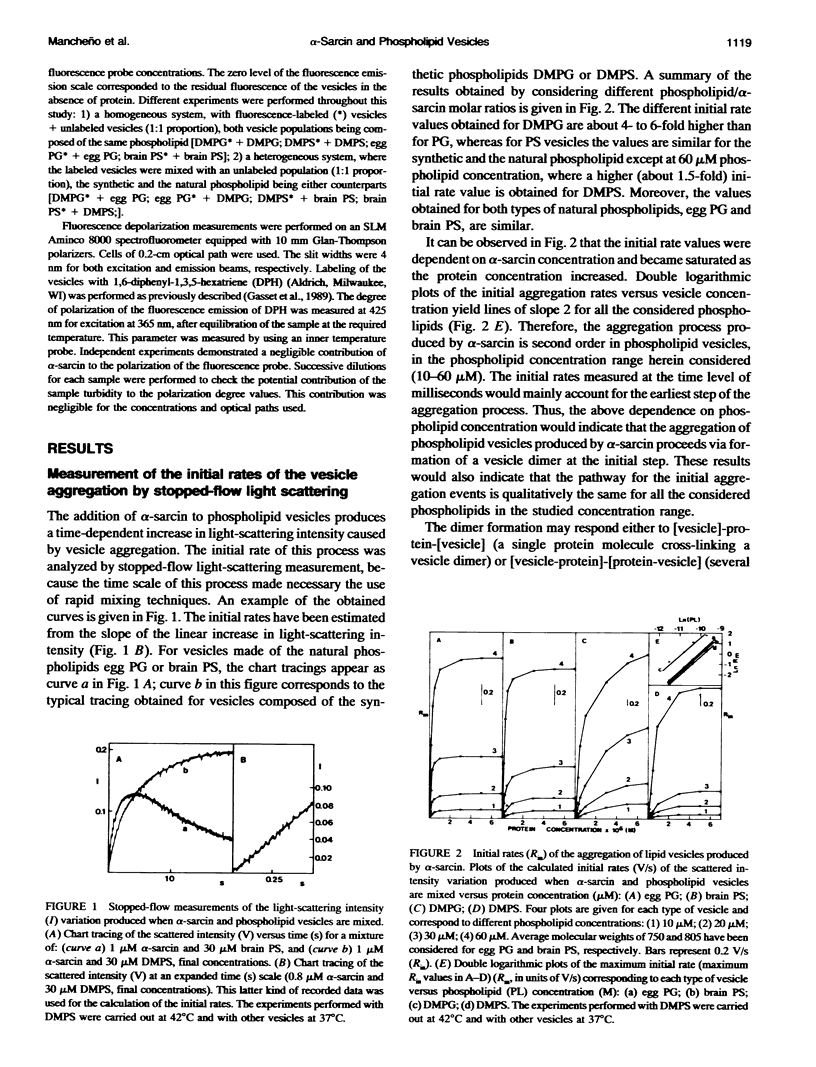
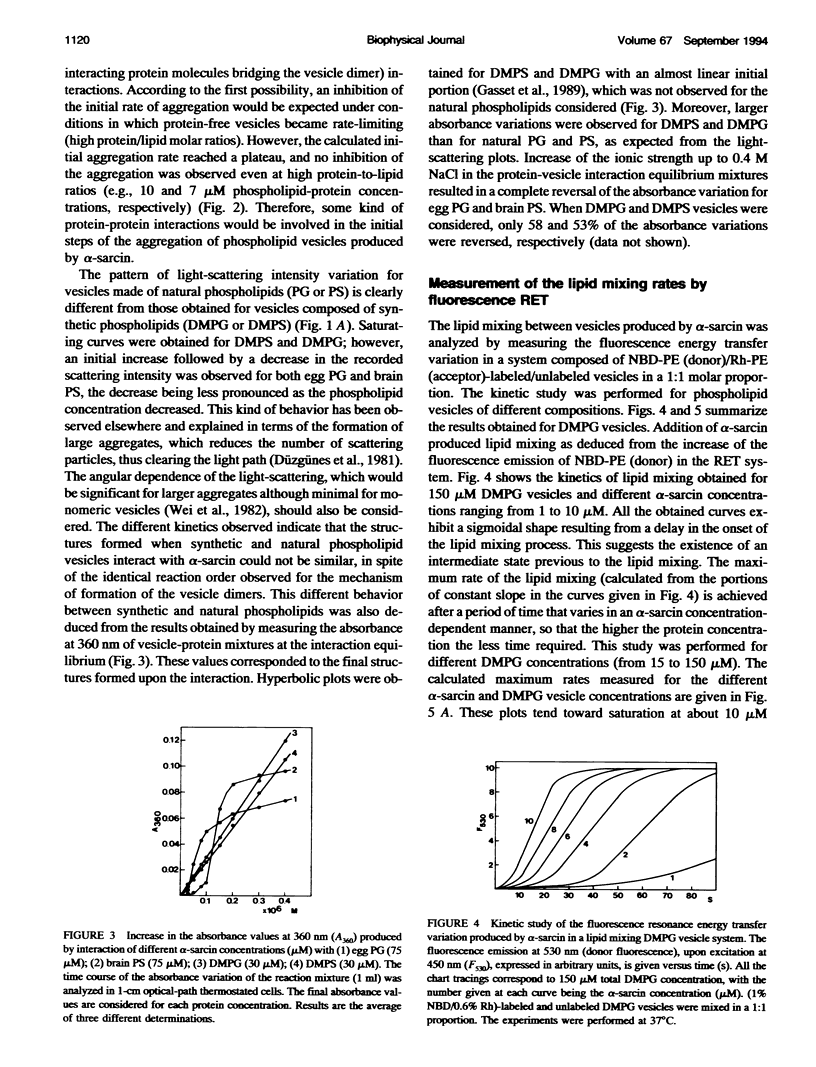
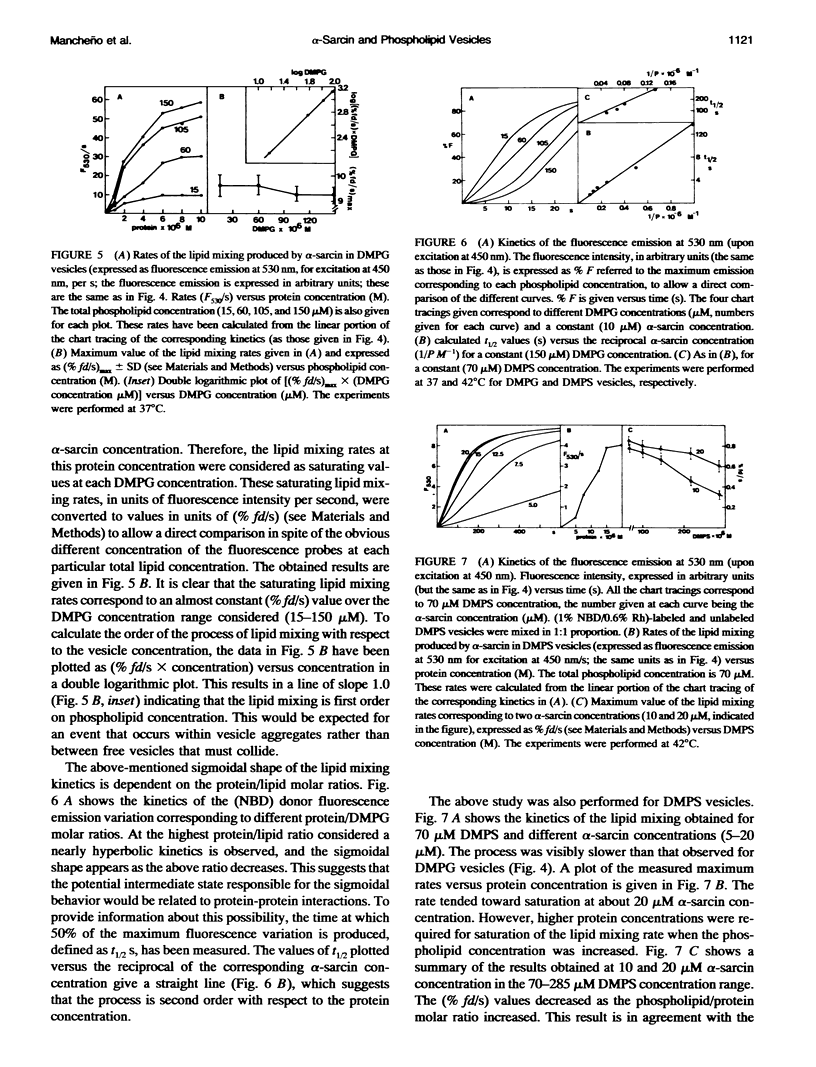
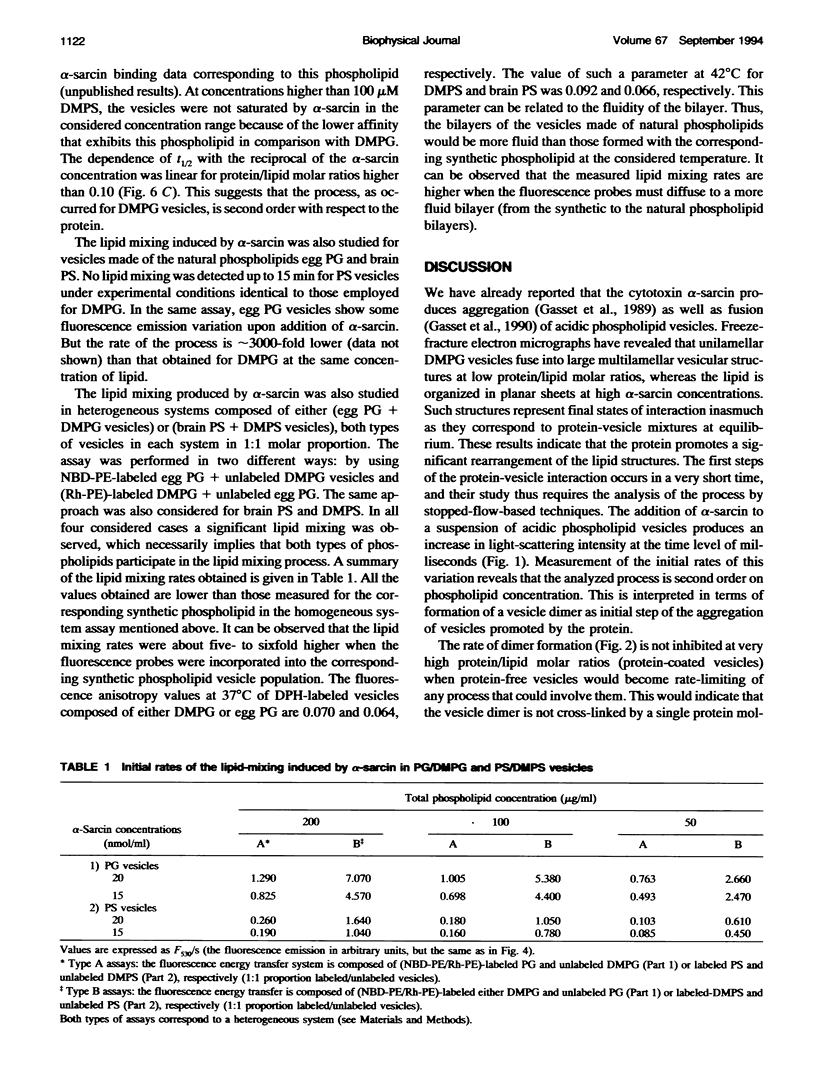
alpha-Sarcin is a fungal cytotoxic protein that inactivates the eukaryotic ribosomes. A kinetic study of the aggregation and lipid mixing promoted by this protein on phosphatidylglycerol (PG) and phosphatidylserine (PS) vesicles has been performed. Egg yolk PG, bovine brain PS, dimyristoyl-PG (DMPG) and dimyristoyl-PS (DMPS) vesicles have been considered. The initial rates of the vesicle aggregation induced by the protein have been measured by stopped-flow 90 degrees light scattering. The formation of a vesicle dimer as the initial step of this process was deduced from the second-order dependence of the initial rates on phospholipid concentration. The highest alpha-sarcin concentration studied did not inhibit the vesicle aggregation, indicating that many protein molecules are involved in the vesicle cross-linking. These are common characteristics of the initial steps of the aggregation produced by alpha-sarcin in the four types of phospholipid vesicles considered. However, the kinetics of the scattering values revealed that more complex changes occurred in the later steps of the aggregation process of egg PG and brain PS vesicles than in those of their synthetic counterparts. alpha-Sarcin produced lipid mixing in vesicles composed of DMPG or DMPS, which was measured by fluorescence resonance energy transfer assays. A delay in the onset of the process, dependent on the protein concentration, was observed. Measurement of the rates of lipid mixing revealed that the process is first order on phospholipid concentration. Egg PG and brain PS vesicles did not show lipid mixing, although they avidly aggregated. However, alpha-sarcin was able to promote lipid mixing in heterogeneous systems composed of egg PG+DMPG or brain PS+DMPS vesicles. The dilution of the fluorescence probes was faster when these were incorporated into the bilayers made of synthetic phospholipids than were present in those made of natural phospholipids. The bilayer destabilization produced by the protein in the vesices composed of the dimyristoyl-phospholipids should be transmitted to the more stable ones made of natural phospholipids. The obtained results are interpreted in terms of lipid mixing occurring within vesicle aggregates larger than dimer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Demel R. A., Paltauf F., Hauser H. Monolayer characteristics and thermal behavior of natural and synthetic phosphatidylserines. Biochemistry. 1987 Dec 29;26(26):8659–8665. doi: 10.1021/bi00400a025. [DOI] [PubMed] [Google Scholar]
- Feigenson G. W. On the nature of calcium ion binding between phosphatidylserine lamellae. Biochemistry. 1986 Sep 23;25(19):5819–5825. doi: 10.1021/bi00367a071. [DOI] [PubMed] [Google Scholar]
- Fernández-Puentes C., Carrasco L. Viral infection permeabilizes mammalian cells to protein toxins. Cell. 1980 Jul;20(3):769–775. doi: 10.1016/0092-8674(80)90323-2. [DOI] [PubMed] [Google Scholar]
- Gasset M., Martinez del Pozo A., Oñaderra M., Gavilanes J. G. Study of the interaction between the antitumour protein alpha-sarcin and phospholipid vesicles. Biochem J. 1989 Mar 1;258(2):569–575. doi: 10.1042/bj2580569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasset M., Oñaderra M., Goormaghtigh E., Gavilanes J. G. Acid phospholipid vesicles produce conformational changes on the antitumour protein alpha-sarcin. Biochim Biophys Acta. 1991 Oct 11;1080(1):51–58. doi: 10.1016/0167-4838(91)90111-c. [DOI] [PubMed] [Google Scholar]
- Gasset M., Oñaderra M., Martínez del Pozo A., Schiavo G. P., Laynez J., Usobiaga P., Gavilanes J. G. Effect of the antitumour protein alpha-sarcin on the thermotropic behaviour of acid phospholipid vesicles. Biochim Biophys Acta. 1991 Sep 10;1068(1):9–16. doi: 10.1016/0005-2736(91)90055-d. [DOI] [PubMed] [Google Scholar]
- Gasset M., Oñaderra M., Thomas P. G., Gavilanes J. G. Fusion of phospholipid vesicles produced by the anti-tumour protein alpha-sarcin. Biochem J. 1990 Feb 1;265(3):815–822. doi: 10.1042/bj2650815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilanes J. G., Lizarbe M. A., Municio A. M., Oñaderra M. Fluorescence studies on the lipoprotein complex of the fatty acid synthetase from the insect Ceratitis capitata. Biochemistry. 1981 Sep 29;20(20):5689–5694. doi: 10.1021/bi00523a008. [DOI] [PubMed] [Google Scholar]
- Hübner W., Mantsch H. H., Paltauf F., Hauser H. Conformation of phosphatidylserine in bilayers as studied by Fourier transform infrared spectroscopy. Biochemistry. 1994 Jan 11;33(1):320–326. doi: 10.1021/bi00167a042. [DOI] [PubMed] [Google Scholar]
- Lampe P. D., Wei G. J., Nelsestuen G. L. Stopped-flow studies of myelin basic protein association with phospholipid vesicles and subsequent vesicle aggregation. Biochemistry. 1983 Mar 29;22(7):1594–1599. doi: 10.1021/bi00276a011. [DOI] [PubMed] [Google Scholar]
- Lamy B., Davies J. Isolation and nucleotide sequence of the Aspergillus restrictus gene coding for the ribonucleolytic toxin restrictocin and its expression in Aspergillus nidulans: the leader sequence protects producing strains from suicide. Nucleic Acids Res. 1991 Mar 11;19(5):1001–1006. doi: 10.1093/nar/19.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckband D. E., Helm C. A., Israelachvili J. Role of calcium in the adhesion and fusion of bilayers. Biochemistry. 1993 Feb 2;32(4):1127–1140. doi: 10.1021/bi00055a019. [DOI] [PubMed] [Google Scholar]
- Nir S., Wilschut J., Bentz J. The rate of fusion of phospholipid vesicles and the role of bilayer curvature. Biochim Biophys Acta. 1982 May 21;688(1):275–278. doi: 10.1016/0005-2736(82)90604-6. [DOI] [PubMed] [Google Scholar]
- Oñaderra M., Mancheño J. M., Gasset M., Lacadena J., Schiavo G., Martínez del Pozo A., Gavilanes J. G. Translocation of alpha-sarcin across the lipid bilayer of asolectin vesicles. Biochem J. 1993 Oct 1;295(Pt 1):221–225. doi: 10.1042/bj2950221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco G., Drickamer K., Wool I. G. The primary structure of the cytotoxin alpha-sarcin. J Biol Chem. 1983 May 10;258(9):5811–5818. [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 1980 Dec 23;19(26):6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]


