Abstract
A novel model lipid bilayer membrane is prepared by the addition of phospholipid vesicles to alkanethiol monolayers on gold. This supported hybrid bilayer membrane is rugged, easily and reproducibly prepared in the absence of organic solvent, and is stable for very long periods of time. We have characterized the insulating characteristics of this membrane by examining the rate of electron transfer and by impedance spectroscopy. Supported hybrid bilayers formed from phospholipids and alkanethiols are pinhole-free and demonstrate measured values of conductivity and resistivity which are within an order of magnitude of that reported for black lipid membranes. Capacitance values suggest a dielectric constant of 2.7 for phospholipid membranes in the absence of organic solvent. The protein toxin, melittin, destroys the insulating capability of the phospholipid layer without significantly altering the bilayer structure. This model membrane will allow the assessment of the effect of lipid membrane perturbants on the insulating properties of natural lipid membranes.
Full text
PDF
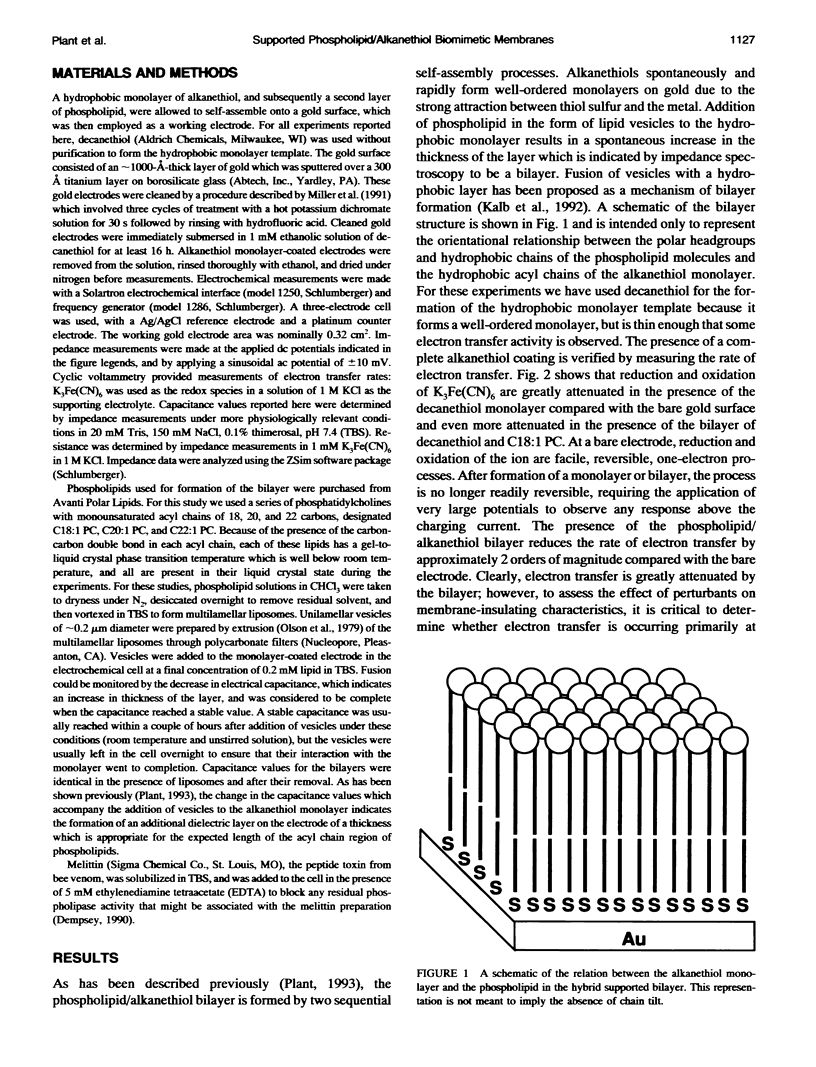
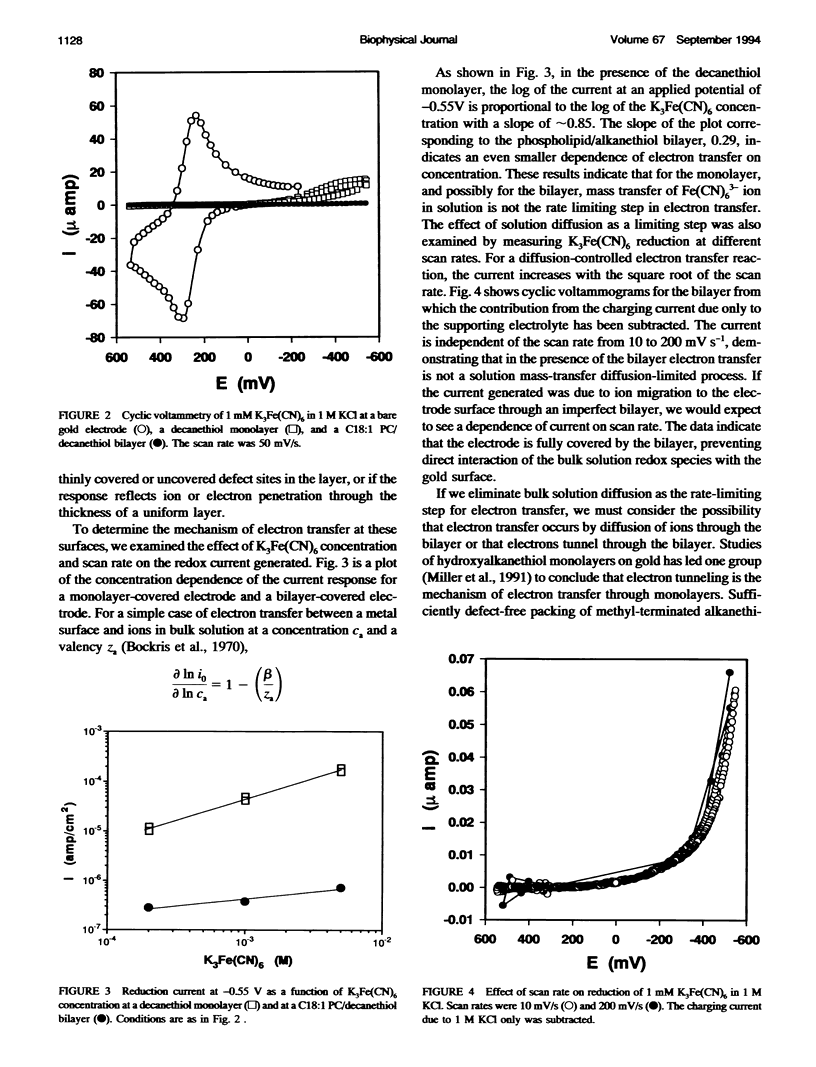
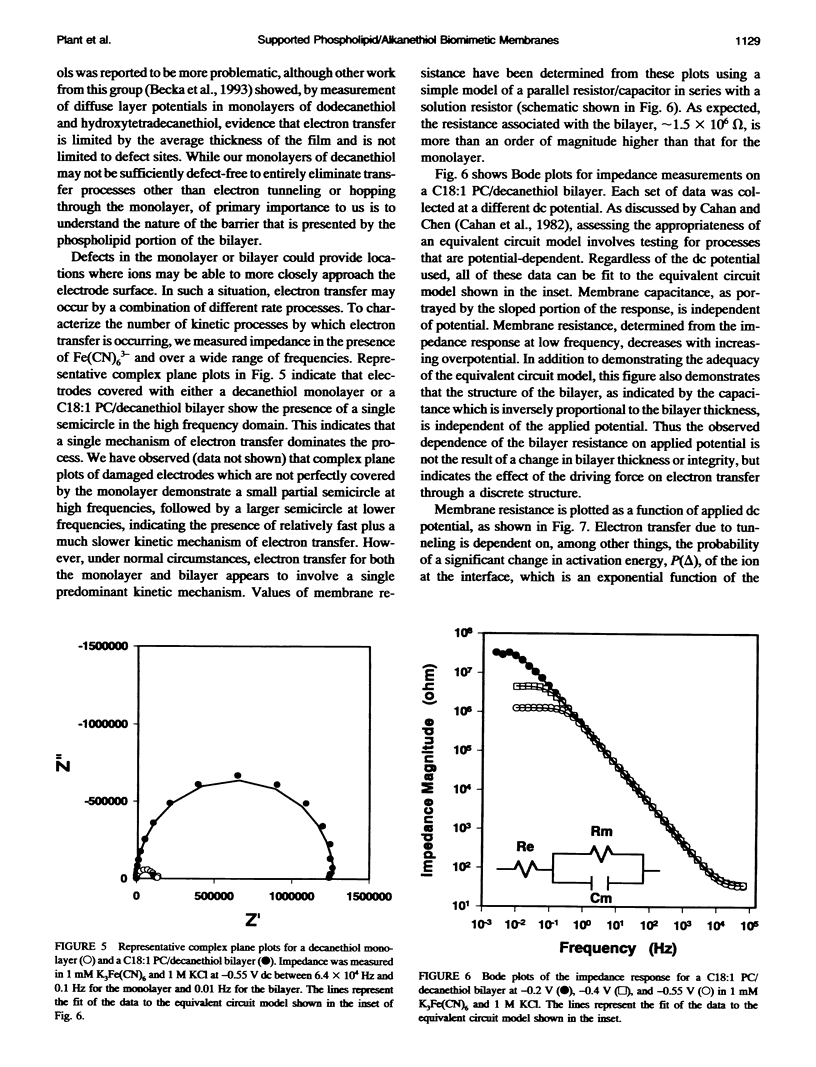
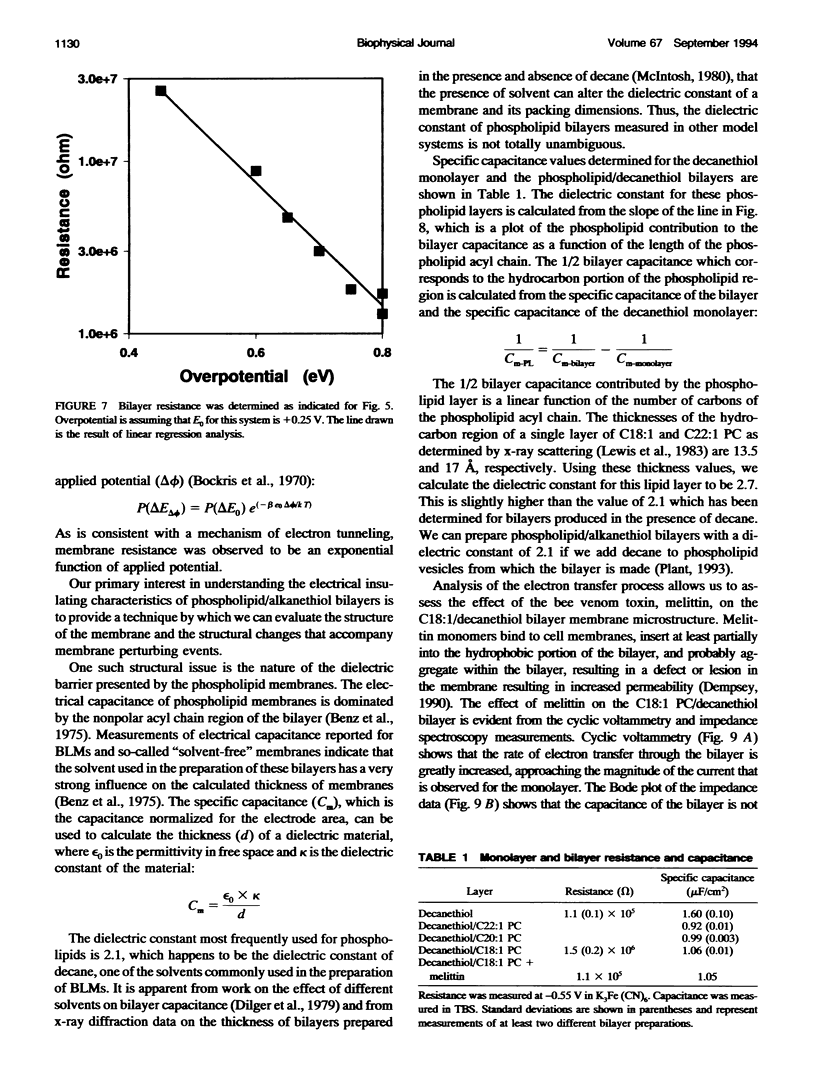


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Fröhlich O., Läuger P., Montal M. Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim Biophys Acta. 1975 Jul 3;394(3):323–334. doi: 10.1016/0005-2736(75)90287-4. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E. The actions of melittin on membranes. Biochim Biophys Acta. 1990 May 7;1031(2):143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- Dilger J. P., McLaughlin S. G., McIntosh T. J., Simon S. A. The dielectric constant of phospholipid bilayers and the permeability of membranes to ions. Science. 1979 Dec 7;206(4423):1196–1198. doi: 10.1126/science.228394. [DOI] [PubMed] [Google Scholar]
- Florin E. L., Gaub H. E. Painted supported lipid membranes. Biophys J. 1993 Feb;64(2):375–383. doi: 10.1016/S0006-3495(93)81378-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb E., Frey S., Tamm L. K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim Biophys Acta. 1992 Jan 31;1103(2):307–316. doi: 10.1016/0005-2736(92)90101-q. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Olson F., Hunt C. A., Szoka F. C., Vail W. J., Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta. 1979 Oct 19;557(1):9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- Spinke J., Yang J., Wolf H., Liley M., Ringsdorf H., Knoll W. Polymer-supported bilayer on a solid substrate. Biophys J. 1992 Dec;63(6):1667–1671. doi: 10.1016/S0006-3495(92)81742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


