Abstract
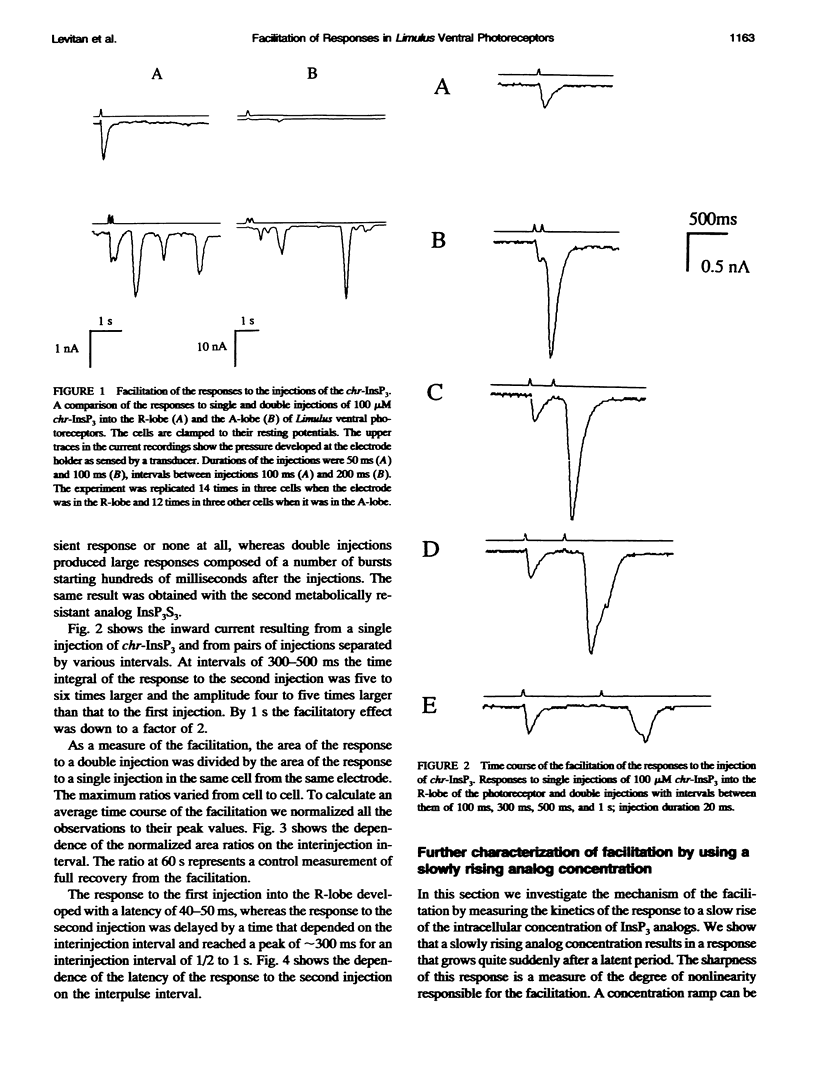
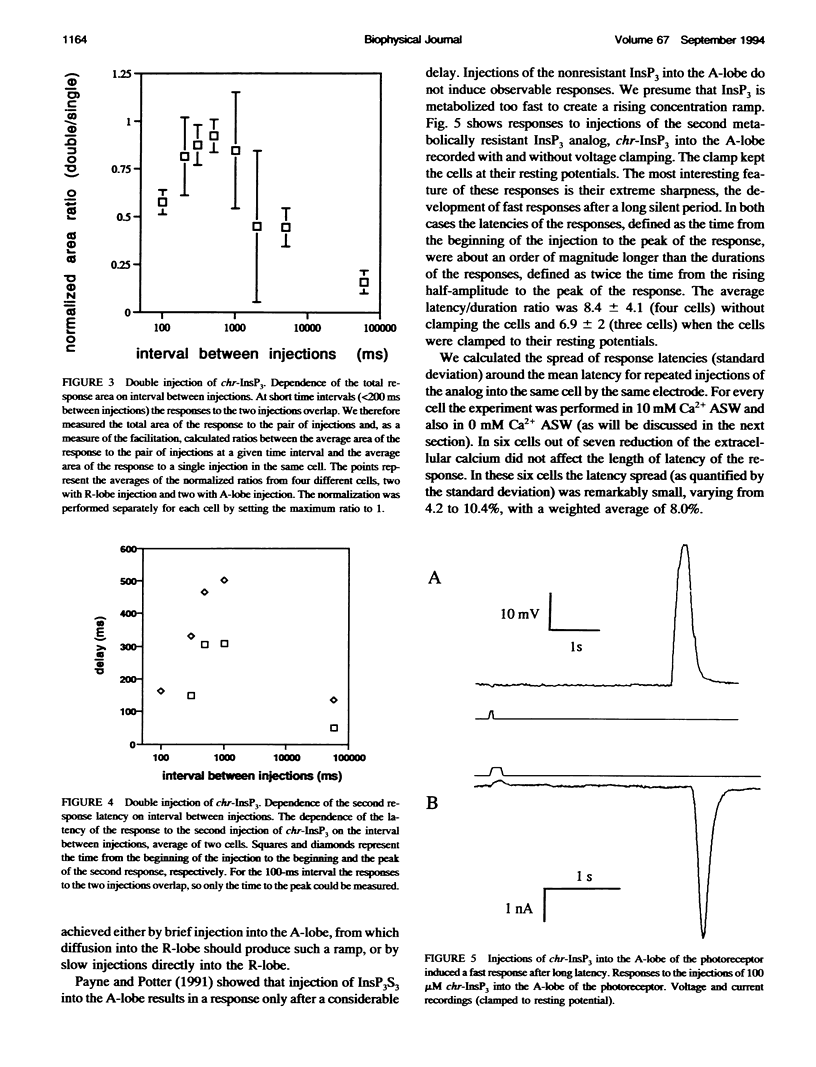
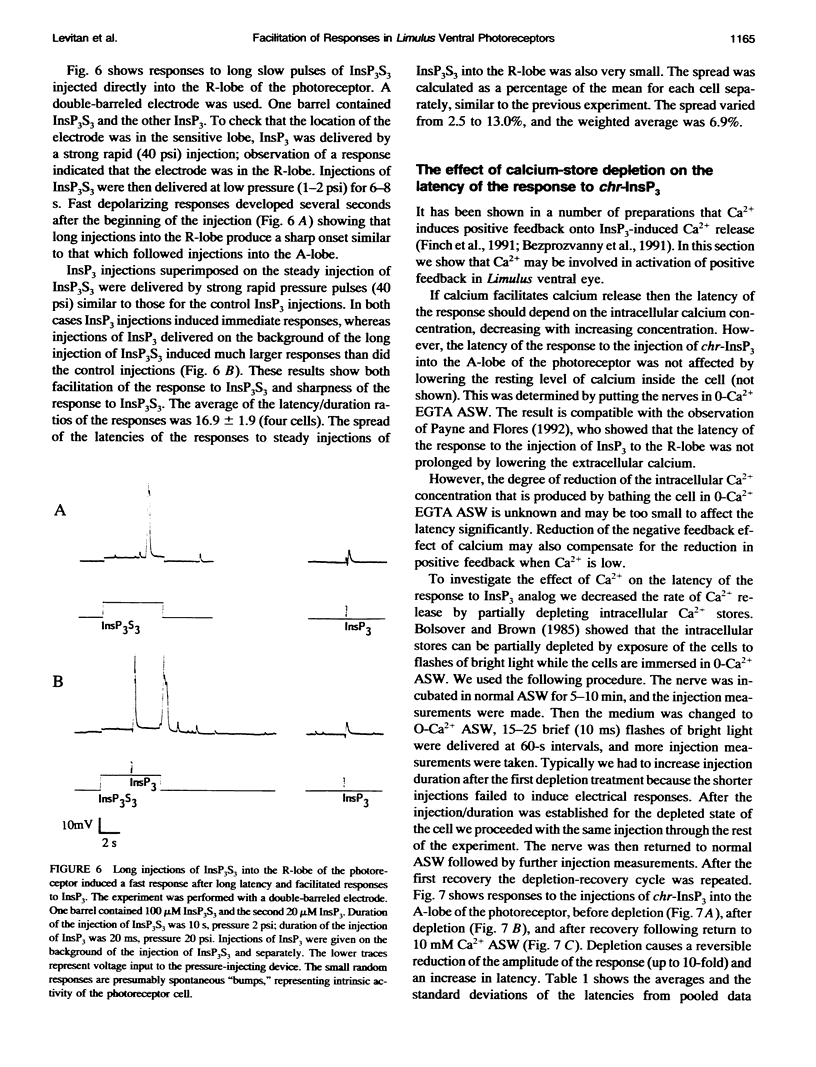
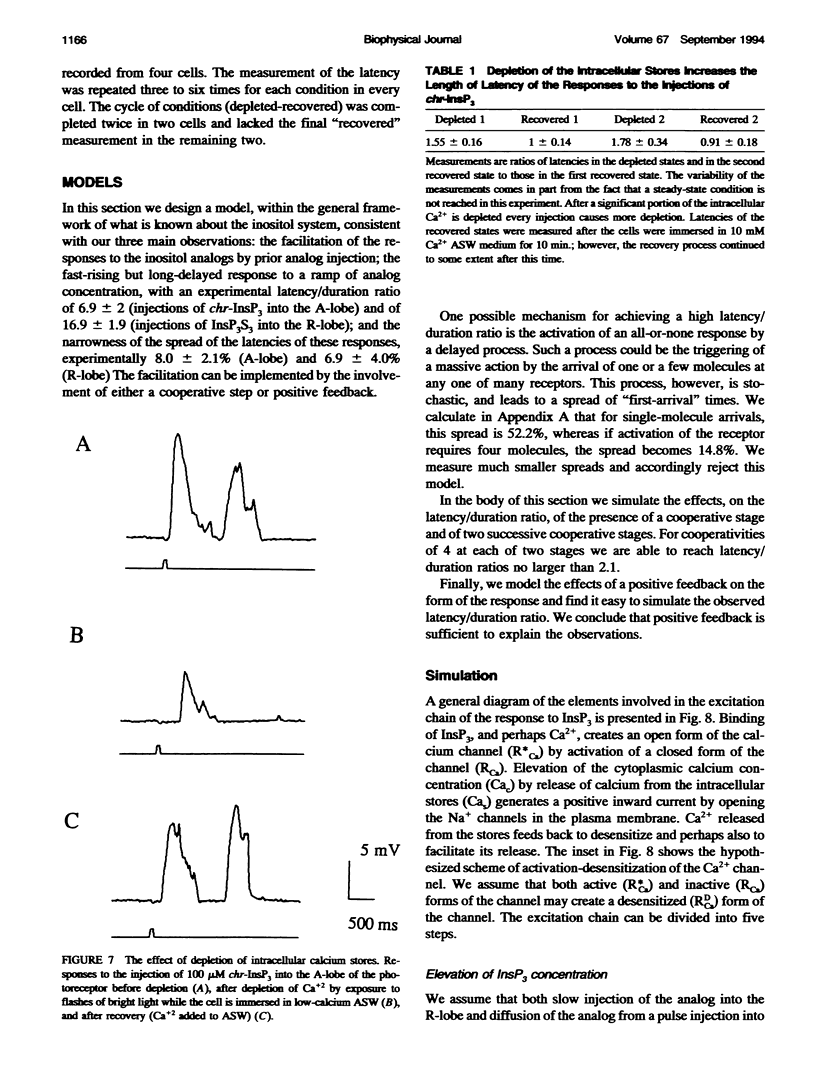
Injection of inositol 1,4,5-trisphosphate and its metabolically resistant analogs InsP3S3 and L-chiro-2,3,5-InsP3 into the ventral photoreceptors of Limulus results in the release of calcium from internal stores and in a current flow into the cells. We show here that the dependence of the current response on the amount of analog injected is supralinear. The injections also facilitate the responses to subsequent injections. We analyze the kinetics of the responses either by very slow application of the analogs directly into the lobe that is sensitive to InsP3 and light or by delivering a pulse into the nonsensitive lobe of the cell, in both cases creating a ramp of rising concentration in the sensitive region. Typically, a long latent period was followed by a strong brief inward current. The ratio between the latency and the duration of the response, defined as twice the time from half-amplitude to the peak of the response, reaches values greater than 10. Our analysis shows that this value cannot be attained within realistic models whose only nonlinearity is the cooperative binding of the ligand to its receptor. The observed ratio, however, can be achieved with a positive feedback model. Treatments that lead to partial depletion of calcium stores reversibly increase the latency of the response. We conclude that the mechanisms of the response of Limulus ventral eye to the metabolically resistant analogs of InsP3 probably involves a positive feedback mechanism and that the carrier of the feedback is likely to be Ca2+.
Full text
PDF

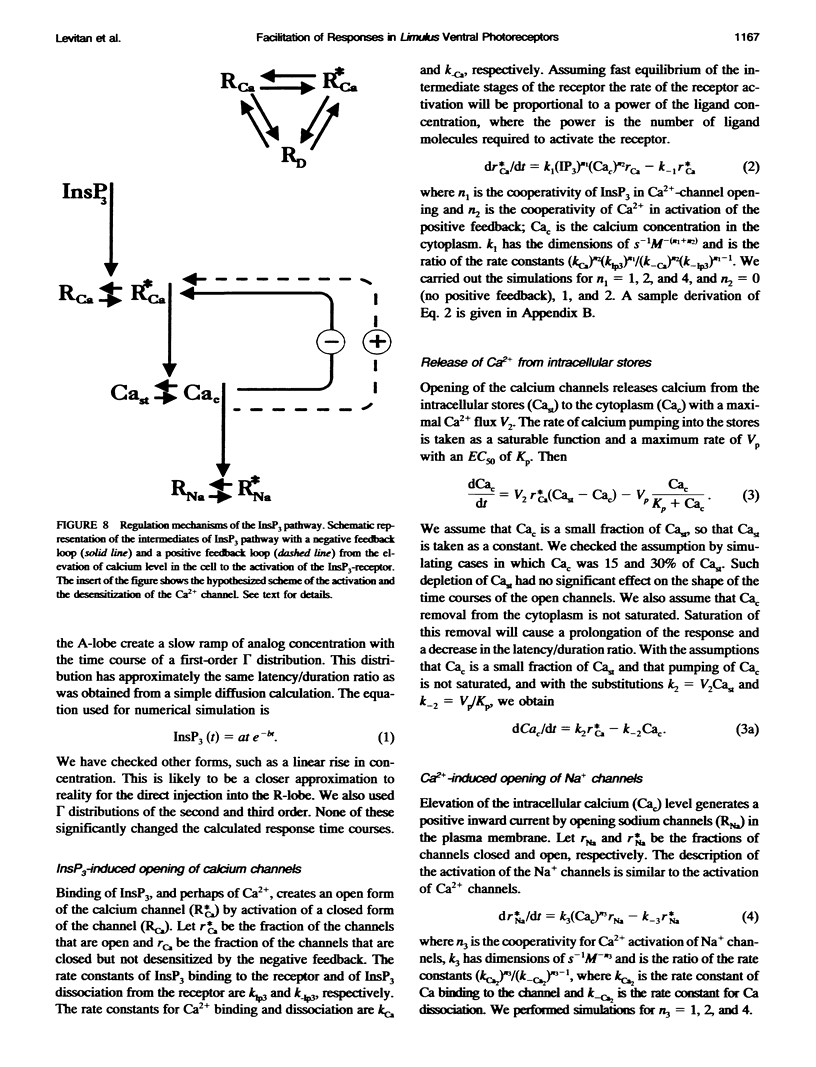

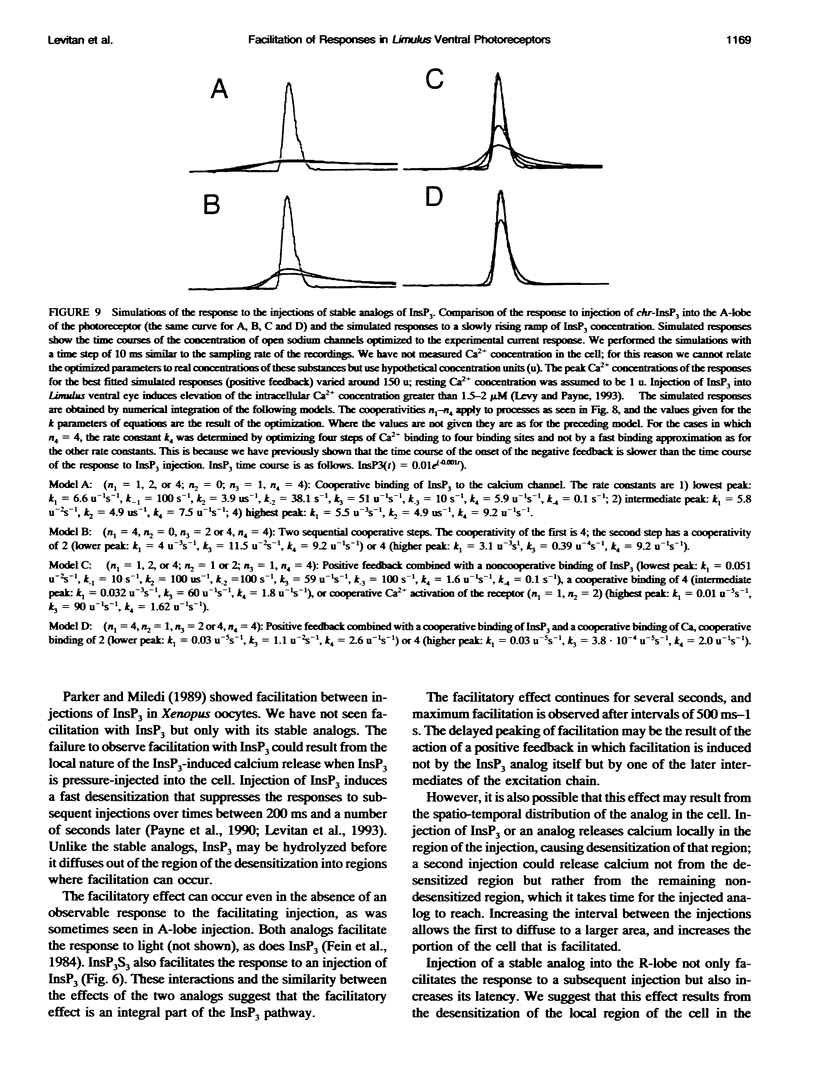



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke A. M., Nahorski S. R., Potter B. V. myo-inositol 1,4,5-trisphosphorothioate is a potent competitive inhibitor of human erythrocyte 5-phosphatase. FEBS Lett. 1989 Jan 2;242(2):373–377. doi: 10.1016/0014-5793(89)80504-6. [DOI] [PubMed] [Google Scholar]
- Corson D. W., Fein A. Inositol 1,4,5-trisphosphate induces bursts of calcium release inside Limulus ventral photoreceptors. Brain Res. 1987 Oct 13;423(1-2):343–346. doi: 10.1016/0006-8993(87)90860-2. [DOI] [PubMed] [Google Scholar]
- Corson D. W., Fein A. Quantitative pressure injection of picoliter volumes into Limulus ventral photoreceptors. Biophys J. 1983 Dec;44(3):299–304. doi: 10.1016/S0006-3495(83)84303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Payne R., Corson D. W., Berridge M. J., Irvine R. F. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature. 1984 Sep 13;311(5982):157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Katz G. M., Schwartz T. L. Temporal control of voltage-clamped membranes: an examination of principles. J Membr Biol. 1974 Jul 12;17(3):275–291. doi: 10.1007/BF01870188. [DOI] [PubMed] [Google Scholar]
- Levitan I., Hillman P., Payne R. Fast desensitization of the response to InsP3 in Limulus ventral photoreceptors. Biophys J. 1993 Apr;64(4):1354–1360. doi: 10.1016/S0006-3495(93)81470-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Payne R., Corson D. W., Fein A., Berridge M. J. Excitation and adaptation of Limulus ventral photoreceptors by inositol 1,4,5 triphosphate result from a rise in intracellular calcium. J Gen Physiol. 1986 Jul;88(1):127–142. doi: 10.1085/jgp.88.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Corson D. W., Fein A. Pressure injection of calcium both excites and adapts Limulus ventral photoreceptors. J Gen Physiol. 1986 Jul;88(1):107–126. doi: 10.1085/jgp.88.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Fein A. Inositol 1,4,5 trisphosphate releases calcium from specialized sites within Limulus photoreceptors. J Cell Biol. 1987 Apr;104(4):933–937. doi: 10.1083/jcb.104.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Flores T. M., Fein A. Feedback inhibition by calcium limits the release of calcium by inositol trisphosphate in Limulus ventral photoreceptors. Neuron. 1990 Apr;4(4):547–555. doi: 10.1016/0896-6273(90)90112-s. [DOI] [PubMed] [Google Scholar]
- Payne R., Flores T. M. The latency of the response of Limulus photoreceptors to inositol trisphosphate lacks the calcium-sensitivity of that to light. J Comp Physiol A. 1992 Mar;170(3):311–316. doi: 10.1007/BF00191419. [DOI] [PubMed] [Google Scholar]
- Payne R., Potter B. V. Injection of inositol trisphosphorothioate into Limulus ventral photoreceptors causes oscillations of free cytosolic calcium. J Gen Physiol. 1991 Jun;97(6):1165–1186. doi: 10.1085/jgp.97.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safrany S. T., Wilcox R. A., Liu C., Potter B. V., Nahorski S. R. 3-position modification of myo-inositol 1,4,5-trisphosphate: consequences for intracellular Ca2+ mobilisation and enzyme recognition. Eur J Pharmacol. 1992 Jul 1;226(3):265–272. doi: 10.1016/0922-4106(92)90071-3. [DOI] [PubMed] [Google Scholar]
- Safrany S. T., Wojcikiewicz R. J., Strupish J., McBain J., Cooke A. M., Potter B. V., Nahorski S. R. Synthetic phosphorothioate-containing analogues of inositol 1,4,5-trisphosphate mobilize intracellular Ca2+ stores and interact differentially with inositol 1,4,5-trisphosphate 5-phosphatase and 3-kinase. Mol Pharmacol. 1991 Jun;39(6):754–761. [PubMed] [Google Scholar]
- Smith T. G., Jr, Barker J. L., Smith B. M., Colburn T. R. Voltage clamping with microelectrodes. J Neurosci Methods. 1980 Dec;3(2):105–128. doi: 10.1016/0165-0270(80)90020-5. [DOI] [PubMed] [Google Scholar]
- Spät A., Bradford P. G., McKinney J. S., Rubin R. P., Putney J. W., Jr A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986 Feb 6;319(6053):514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- Stern J., Chinn K., Bacigalupo J., Lisman J. Distinct lobes of Limulus ventral photoreceptors. I. Functional and anatomical properties of lobes revealed by removal of glial cells. J Gen Physiol. 1982 Dec;80(6):825–837. doi: 10.1085/jgp.80.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Isla B. A., Alcayaga C., Marengo J. J., Bull R. Activation of inositol trisphosphate-sensitive Ca2+ channels of sarcoplasmic reticulum from frog skeletal muscle. J Physiol. 1991 Sep;441:575–591. doi: 10.1113/jphysiol.1991.sp018768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Isla B. A., Irribarra V., Oberhauser A., Larralde L., Bull R., Hidalgo C., Jaimovich E. Inositol (1,4,5)-trisphosphate activates a calcium channel in isolated sarcoplasmic reticulum membranes. Biophys J. 1988 Oct;54(4):737–741. doi: 10.1016/S0006-3495(88)83009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras J., Bezprozvanny I., Ehrlich B. E. Inositol 1,4,5-trisphosphate-gated channels in cerebellum: presence of multiple conductance states. J Neurosci. 1991 Oct;11(10):3239–3245. doi: 10.1523/JNEUROSCI.11-10-03239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. F., Szuts E. Z., Fein A. Metabolism of inositol 1,4,5-trisphosphate in squid photoreceptors. J Comp Physiol B. 1990;160(3):293–298. doi: 10.1007/BF00302595. [DOI] [PubMed] [Google Scholar]


