Abstract
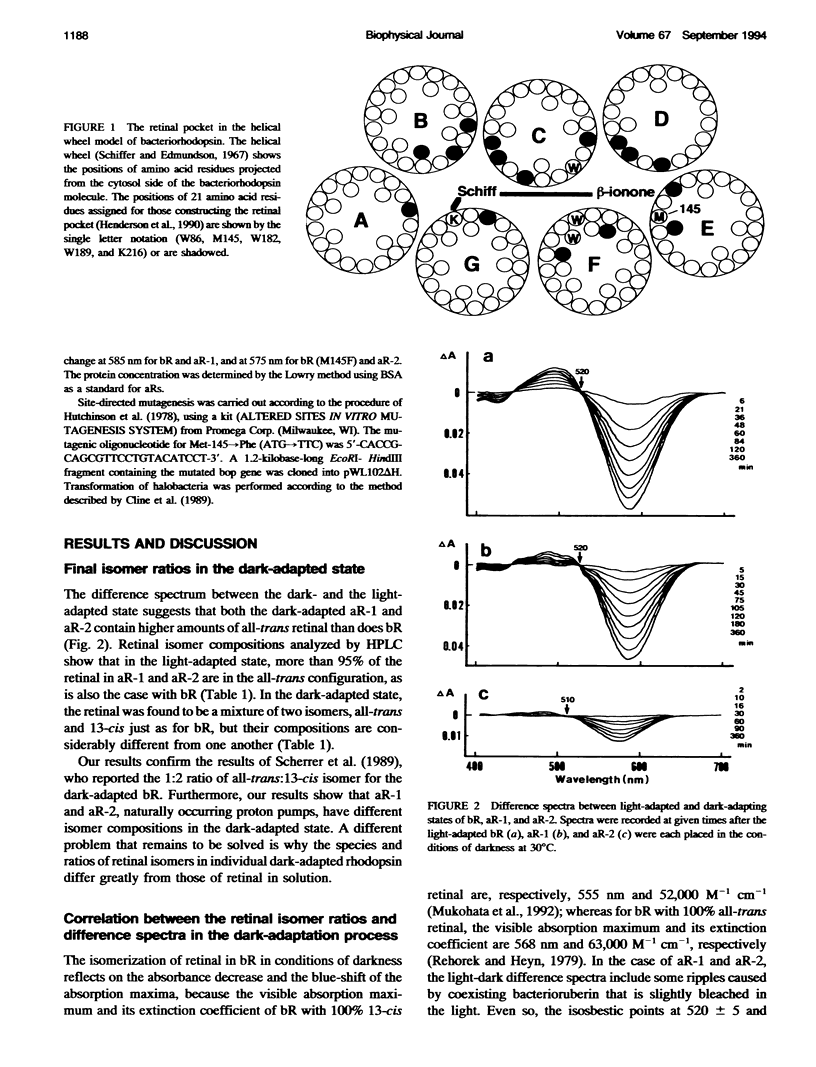
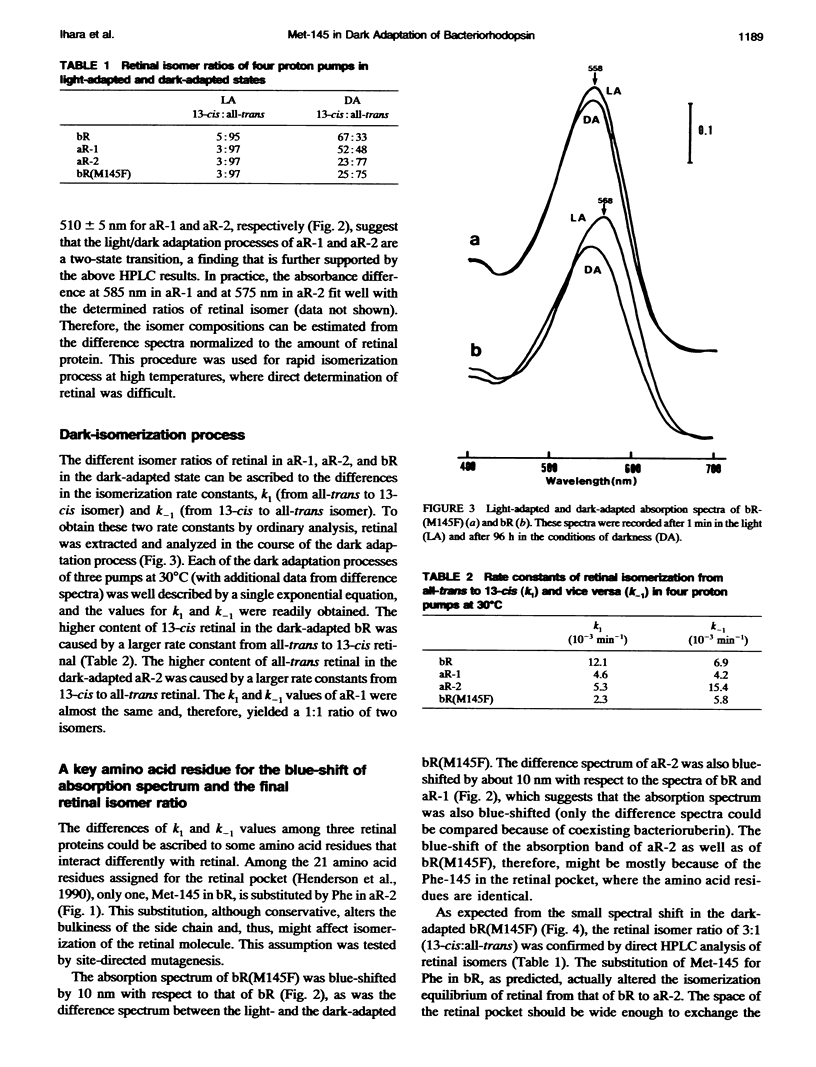
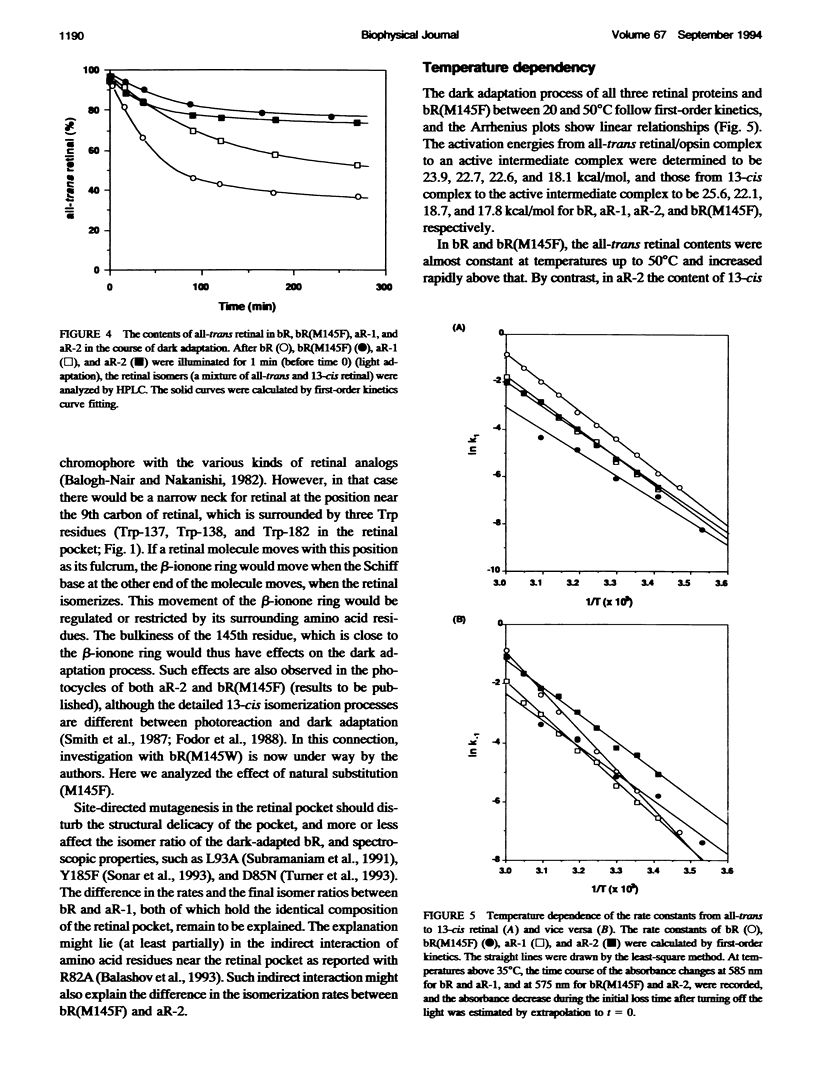
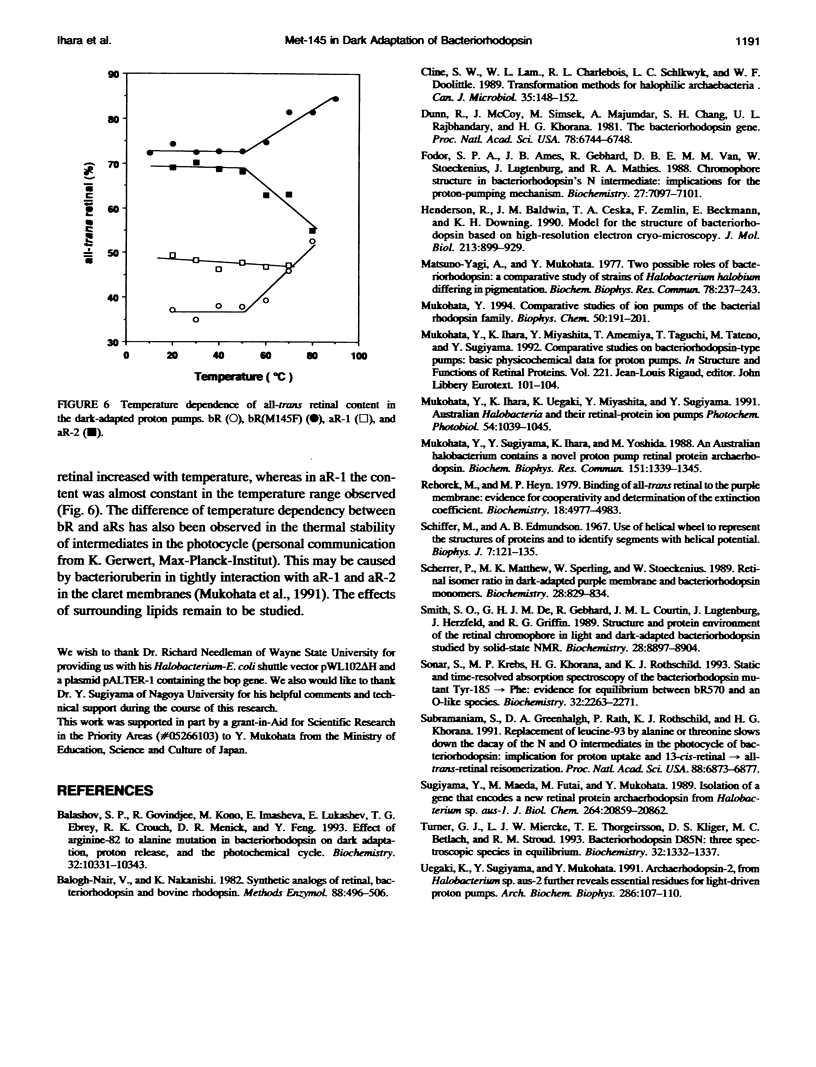
Composition of retinal isomers in three proton pumps (bacteriorhodopsin, archaerhodopsin-1, and archaerhodopsin-2) was determined by high performance liquid chromatography in their light-adapted and dark-adapted states. In the light-adapted state, more than 95% of the retinal in all three proton pumps were in the all-trans configuration. In the dark-adapted state, there were only two retinal isomers, all-trans and 13-cis, in the ratio of all-trans: 13-cis = 1:2 for bacteriorhodopsin, 1:1 for archaerhodopsin-1, and 3:1 for archaerhodopsin-2. The difference in the final isomer ratios in the dark-adapted bacteriorhodopsin and archaerhodopsin-2 was ascribed to the methionine-145 in bacteriorhodopsin. This is the only amino acid in the retinal pocket that is substituted by phenylalanine in archaerhodopsin-2. The bacteriorhodopsin point-mutated at this position to phenylalanine dramatically altered the final isomer ratio from 1:2 to 3:1 in the dark-adapted state. This point mutation also caused a 10 nm blue-shift of the adsorption spectrum, which is similar to the shift of archaerhodopsin-2 relative to the spectra of bacteriorhodopsin and archaerhodopsin-1.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balashov S. P., Govindjee R., Kono M., Imasheva E., Lukashev E., Ebrey T. G., Crouch R. K., Menick D. R., Feng Y. Effect of the arginine-82 to alanine mutation in bacteriorhodopsin on dark adaptation, proton release, and the photochemical cycle. Biochemistry. 1993 Oct 5;32(39):10331–10343. doi: 10.1021/bi00090a008. [DOI] [PubMed] [Google Scholar]
- Dunn R., McCoy J., Simsek M., Majumdar A., Chang S. H., Rajbhandary U. L., Khorana H. G. The bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6744–6748. doi: 10.1073/pnas.78.11.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Leventhal J. M., Hutchinson T. A., Kramer M. S., Feinstein A. R. An algorithm for the operational assessment of adverse drug reactions. III. Results of tests among clinicians. JAMA. 1979 Nov 2;242(18):1991–1994. [PubMed] [Google Scholar]
- Matsuno-Yagi A., Mukohata Y. Two possible roles of bacteriorhodopsin; a comparative study of strains of Halobacterium halobium differing in pigmentation. Biochem Biophys Res Commun. 1977 Sep 9;78(1):237–243. doi: 10.1016/0006-291x(77)91245-1. [DOI] [PubMed] [Google Scholar]
- Mukohata Y. Comparative studies on ion pumps of the bacterial rhodopsin family. Biophys Chem. 1994 May;50(1-2):191–201. doi: 10.1016/0301-4622(94)85031-3. [DOI] [PubMed] [Google Scholar]
- Mukohata Y., Ihara K., Uegaki K., Miyashita Y., Sugiyama Y. Australian Halobacteria and their retinal-protein ion pumps. Photochem Photobiol. 1991 Dec;54(6):1039–1045. doi: 10.1111/j.1751-1097.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- Mukohata Y., Sugiyama Y., Ihara K., Yoshida M. An Australian halobacterium contains a novel proton pump retinal protein: archaerhodopsin. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1339–1345. doi: 10.1016/s0006-291x(88)80509-6. [DOI] [PubMed] [Google Scholar]
- Rehorek M., Heyn M. P. Binding of all-trans-retinal to the purple membrane. Evidence for cooperativity and determination of the extinction coefficient. Biochemistry. 1979 Oct 30;18(22):4977–4983. doi: 10.1021/bi00589a027. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. O., de Groot H. J., Gebhard R., Courtin J. M., Lugtenburg J., Herzfeld J., Griffin R. G. Structure and protein environment of the retinal chromophore in light- and dark-adapted bacteriorhodopsin studied by solid-state NMR. Biochemistry. 1989 Oct 31;28(22):8897–8904. doi: 10.1021/bi00448a032. [DOI] [PubMed] [Google Scholar]
- Sonar S., Krebs M. P., Khorana H. G., Rothschild K. J. Static and time-resolved absorption spectroscopy of the bacteriorhodopsin mutant Tyr-185-->Phe: evidence for an equilibrium between bR570 and an O-like species. Biochemistry. 1993 Mar 9;32(9):2263–2271. doi: 10.1021/bi00060a019. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Greenhalgh D. A., Rath P., Rothschild K. J., Khorana H. G. Replacement of leucine-93 by alanine or threonine slows down the decay of the N and O intermediates in the photocycle of bacteriorhodopsin: implications for proton uptake and 13-cis-retinal----all-trans-retinal reisomerization. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6873–6877. doi: 10.1073/pnas.88.15.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y., Maeda M., Futai M., Mukohata Y. Isolation of a gene that encodes a new retinal protein, archaerhodopsin, from Halobacterium sp. aus-1. J Biol Chem. 1989 Dec 15;264(35):20859–20862. [PubMed] [Google Scholar]
- Turner G. J., Miercke L. J., Thorgeirsson T. E., Kliger D. S., Betlach M. C., Stroud R. M. Bacteriorhodopsin D85N: three spectroscopic species in equilibrium. Biochemistry. 1993 Feb 9;32(5):1332–1337. doi: 10.1021/bi00056a019. [DOI] [PubMed] [Google Scholar]
- Uegaki K., Sugiyama Y., Mukohata Y. Archaerhodopsin-2, from Halobacterium sp. aus-2 further reveals essential amino acid residues for light-driven proton pumps. Arch Biochem Biophys. 1991 Apr;286(1):107–110. doi: 10.1016/0003-9861(91)90014-a. [DOI] [PubMed] [Google Scholar]


