Abstract
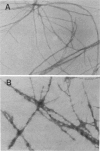
beta-amyloid peptide (A beta) is the major protein component of senile plaques and cerebrovascular amyloid deposits in Alzheimer's patients. Several researchers have demonstrated that A beta is neurotoxic in in vitro and in vivo systems. Peptide aggregation state and/or conformation might play a significant role in determining the toxicity of the peptide. The size and flexibility of fibrils formed from the synthetic peptide beta (1-39), corresponding to the first 39 residues of A beta, were determined. Samples were prepared either directly from lyophilized peptide or diluted from a 10 mg/ml stock solution in 0.1% trifluoroacetic acid (TFA). All samples had a final peptide concentration of 0.5 mg/ml, a final pH of 7.4, and a final NaCl concentration of 0.14 M. The molecular weight and linear density of the fibrils increased with increasing pre-incubation time in TFA, based on static light scattering measurements. Analysis of the angular dependence of the intensity of scattered light indicated that the fibrils were semi-flexible chains and that the fibril flexibility decreased with increasing pre-incubation time in TFA. There was a concomitant change in phase behavior from precipitation to gelation with the decrease in fibril flexibility.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow C. J., Yasuda A., Kenny P. T., Zagorski M. G. Solution conformations and aggregational properties of synthetic amyloid beta-peptides of Alzheimer's disease. Analysis of circular dichroism spectra. J Mol Biol. 1992 Jun 20;225(4):1075–1093. doi: 10.1016/0022-2836(92)90106-t. [DOI] [PubMed] [Google Scholar]
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992 Jan 5;267(1):546–554. [PubMed] [Google Scholar]
- Busciglio J., Lorenzo A., Yankner B. A. Methodological variables in the assessment of beta amyloid neurotoxicity. Neurobiol Aging. 1992 Sep-Oct;13(5):609–612. doi: 10.1016/0197-4580(92)90065-6. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Yeh J., Yankner B. A. beta-Amyloid neurotoxicity in human cortical culture is not mediated by excitotoxins. J Neurochem. 1993 Oct;61(4):1565–1568. doi: 10.1111/j.1471-4159.1993.tb13658.x. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Clemens J. A., Stephenson D. T. Implants containing beta-amyloid protein are not neurotoxic to young and old rat brain. Neurobiol Aging. 1992 Sep-Oct;13(5):581–586. doi: 10.1016/0197-4580(92)90059-7. [DOI] [PubMed] [Google Scholar]
- Emre M., Geula C., Ransil B. J., Mesulam M. M. The acute neurotoxicity and effects upon cholinergic axons of intracerebrally injected beta-amyloid in the rat brain. Neurobiol Aging. 1992 Sep-Oct;13(5):553–559. doi: 10.1016/0197-4580(92)90055-3. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Surewicz W. K., Kirschner D. A. pH-dependent structural transitions of Alzheimer amyloid peptides. Biophys J. 1991 Nov;60(5):1190–1201. doi: 10.1016/S0006-3495(91)82154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy S. A., Baird A., Cole G. M. Effects of injected Alzheimer beta-amyloid cores in rat brain. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8362–8366. doi: 10.1073/pnas.88.19.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D., Khan K. M., Soriano F. G., Keim P. S., Davis D. L., Bryant K., Lieberburg I. Lack of Alzheimer pathology after beta-amyloid protein injections in rat brain. Neurobiol Aging. 1992 Sep-Oct;13(5):569–576. doi: 10.1016/0197-4580(92)90057-5. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993 Dec 17;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Halverson K., Fraser P. E., Kirschner D. A., Lansbury P. T., Jr Molecular determinants of amyloid deposition in Alzheimer's disease: conformational studies of synthetic beta-protein fragments. Biochemistry. 1990 Mar 20;29(11):2639–2644. doi: 10.1021/bi00463a003. [DOI] [PubMed] [Google Scholar]
- Hilbich C., Kisters-Woike B., Reed J., Masters C. L., Beyreuther K. Aggregation and secondary structure of synthetic amyloid beta A4 peptides of Alzheimer's disease. J Mol Biol. 1991 Mar 5;218(1):149–163. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- Jordano J., Barbero J. L., Montero F., Franco L. Fluorescence of histones H1. A tyrosinate-like fluorescence emission in Ceratitis capitata H1 at neutral pH values. J Biol Chem. 1983 Jan 10;258(1):315–320. [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Klunk W. E., Pettegrew J. W., Abraham D. J. Quantitative evaluation of congo red binding to amyloid-like proteins with a beta-pleated sheet conformation. J Histochem Cytochem. 1989 Aug;37(8):1273–1281. doi: 10.1177/37.8.2666510. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Yang L. L., Cotman C. W. Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990 Nov 19;533(2):315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Beal M. F., Busciglio J., Duffy L. K., Yankner B. A. An in vivo model for the neurodegenerative effects of beta amyloid and protection by substance P. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall N. W., McKee A. C., Yankner B. A., Beal M. F. In vivo neurotoxicity of beta-amyloid [beta(1-40)] and the beta(25-35) fragment. Neurobiol Aging. 1992 Sep-Oct;13(5):537–542. doi: 10.1016/0197-4580(92)90053-z. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Loo D. T., Copani A., Pike C. J., Whittemore E. R., Walencewicz A. J., Cotman C. W. Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P. C., Gitter B. D., Waters D. C., Simmons L. K., Becker G. W., Small J. S., Robison P. M. beta-Amyloid peptide in vitro toxicity: lot-to-lot variability. Neurobiol Aging. 1992 Sep-Oct;13(5):605–607. doi: 10.1016/0197-4580(92)90064-5. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Wisniewski H. M., Somerville R. A., Bobin S. A., Masters C. L., Iqbal K. Ultrastructural morphology of amyloid fibrils from neuritic and amyloid plaques. Acta Neuropathol. 1983;60(1-2):113–124. doi: 10.1007/BF00685355. [DOI] [PubMed] [Google Scholar]
- Narang H. K. High-resolution electron microscopic analysis of the amyloid fibril in Alzheimer's disease. J Neuropathol Exp Neurol. 1980 Nov;39(6):621–631. doi: 10.1097/00005072-198011000-00001. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. Aggregation-related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur J Pharmacol. 1991 Aug 14;207(4):367–368. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991 Nov 1;563(1-2):311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- Podlisny M. B., Stephenson D. T., Frosch M. P., Lieberburg I., Clemens J. A., Selkoe D. J. Synthetic amyloid beta-protein fails to produce specific neurotoxicity in monkey cerebral cortex. Neurobiol Aging. 1992 Sep-Oct;13(5):561–567. doi: 10.1016/0197-4580(92)90056-4. [DOI] [PubMed] [Google Scholar]
- Prelli F., Castaño E., Glenner G. G., Frangione B. Differences between vascular and plaque core amyloid in Alzheimer's disease. J Neurochem. 1988 Aug;51(2):648–651. doi: 10.1111/j.1471-4159.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. N. A causal role for amyloid in Alzheimer's disease: the end of the beginning. Neurology. 1993 May;43(5):851–856. doi: 10.1212/wnl.43.5.851. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Simmons L. K., May P. C., Tomaselli K. J., Rydel R. E., Fuson K. S., Brigham E. F., Wright S., Lieberburg I., Becker G. W., Brems D. N. Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol Pharmacol. 1994 Mar;45(3):373–379. [PubMed] [Google Scholar]
- Stein-Behrens B., Adams K., Yeh M., Sapolsky R. Failure of beta-amyloid protein fragment 25-35 to cause hippocampal damage in the rat. Neurobiol Aging. 1992 Sep-Oct;13(5):577–579. doi: 10.1016/0197-4580(92)90058-6. [DOI] [PubMed] [Google Scholar]
- Tomski S. J., Murphy R. M. Kinetics of aggregation of synthetic beta-amyloid peptide. Arch Biochem Biophys. 1992 May 1;294(2):630–638. doi: 10.1016/0003-9861(92)90735-f. [DOI] [PubMed] [Google Scholar]
- Waite J., Cole G. M., Frautschy S. A., Connor D. J., Thal L. J. Solvent effects on beta protein toxicity in vivo. Neurobiol Aging. 1992 Sep-Oct;13(5):595–599. doi: 10.1016/0197-4580(92)90062-3. [DOI] [PubMed] [Google Scholar]
- Whitson J. S., Selkoe D. J., Cotman C. W. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989 Mar 17;243(4897):1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]