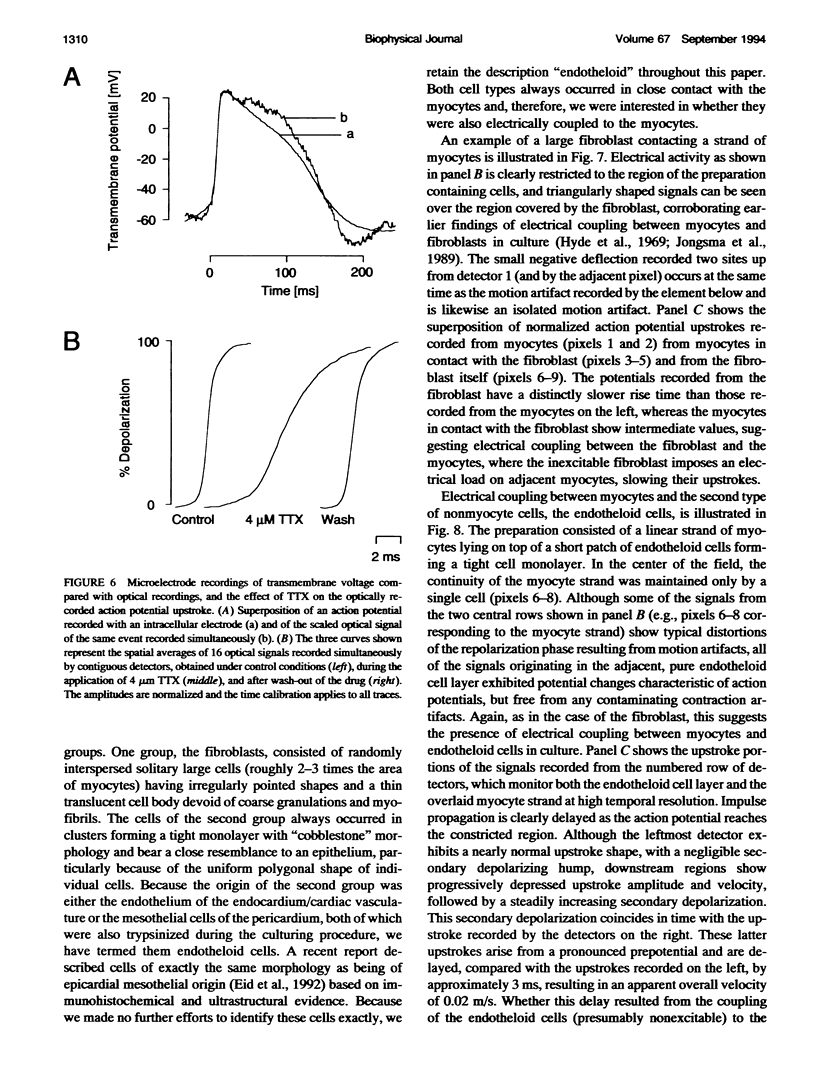
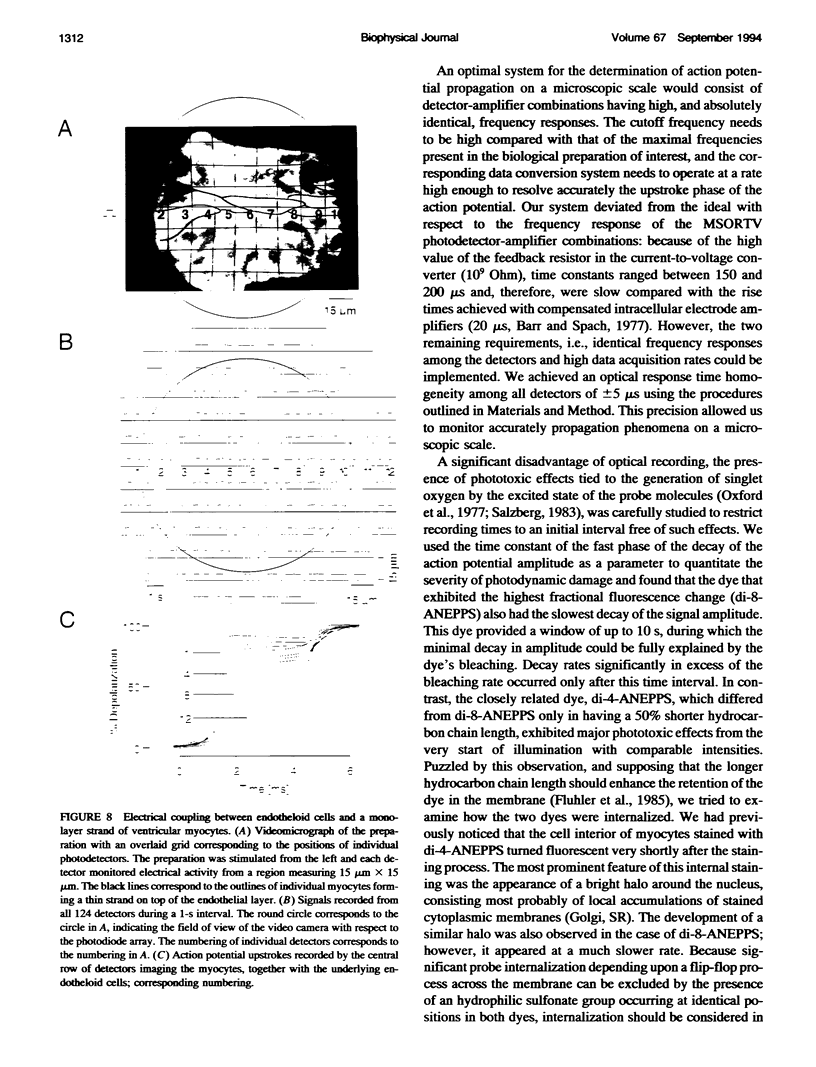
Abstract
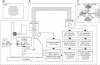
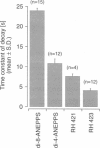
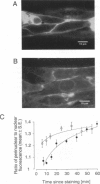
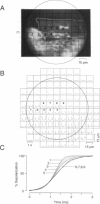
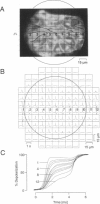
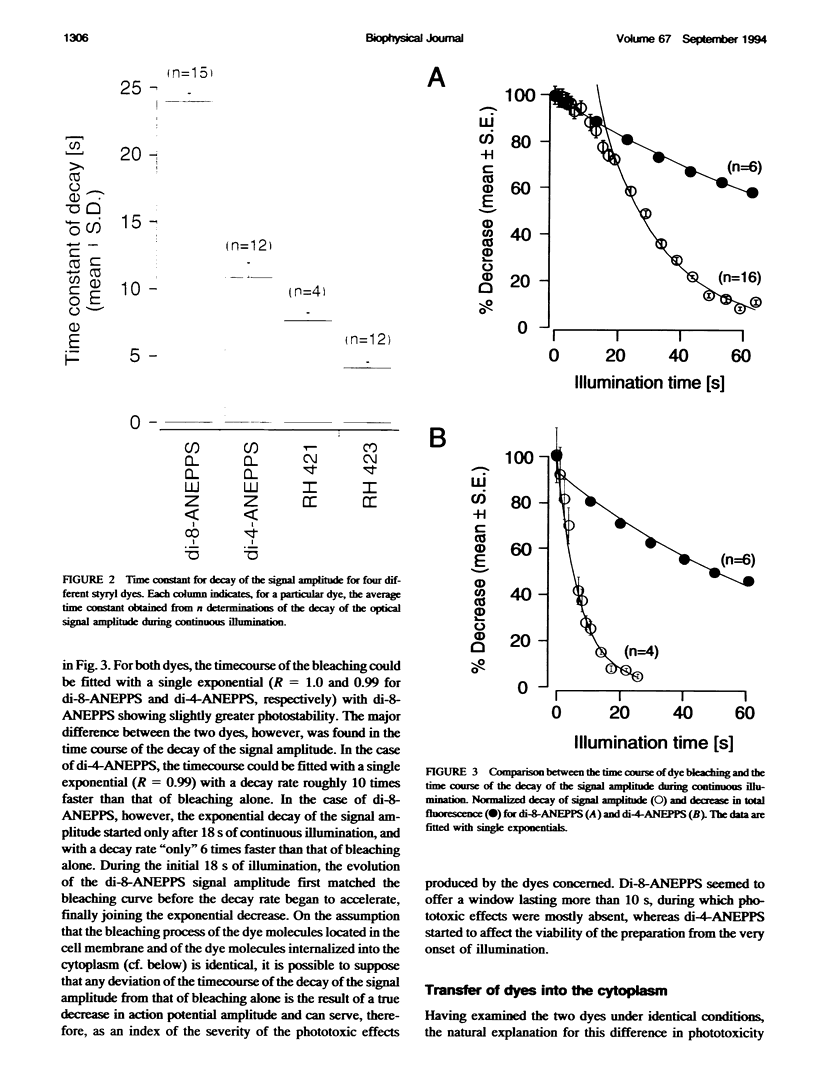
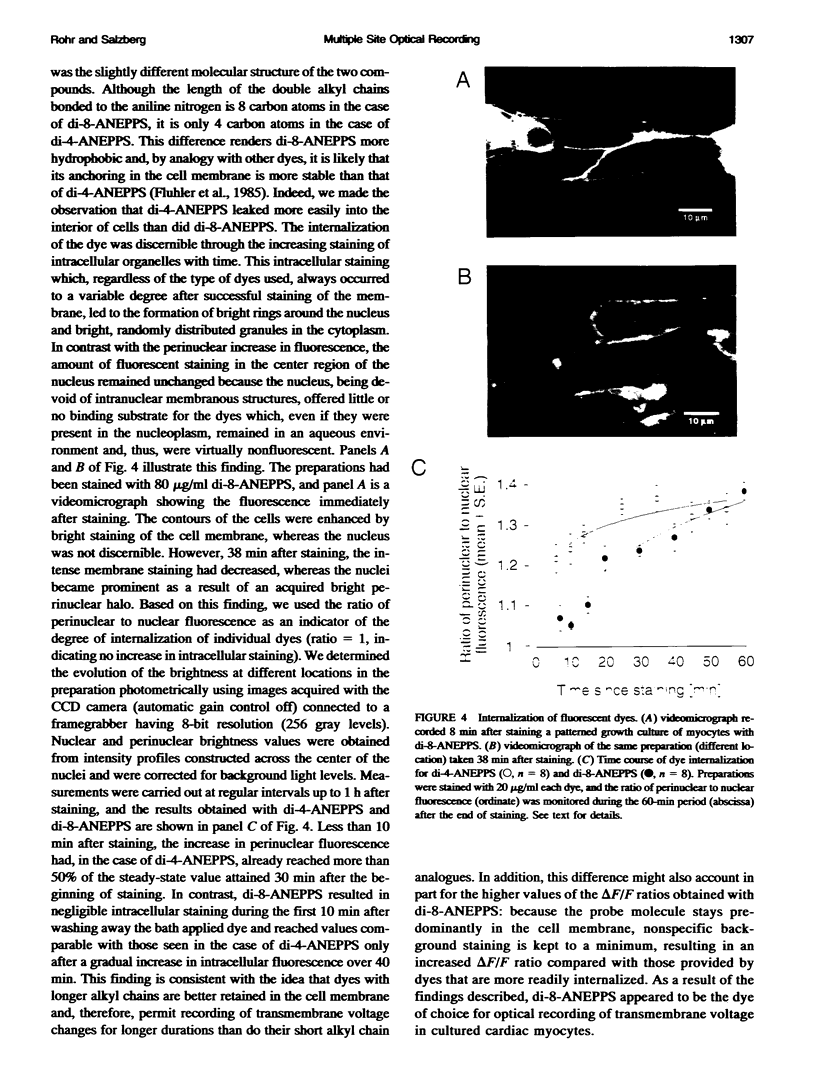
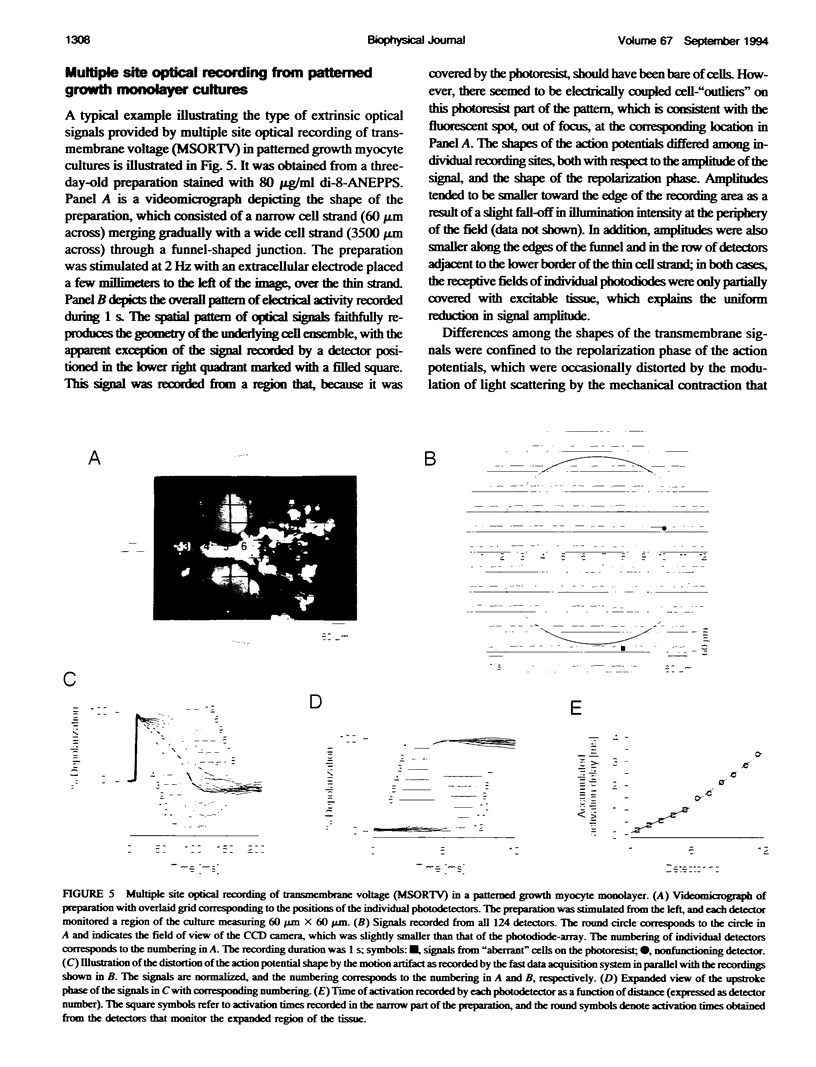
We have applied multiple site optical recording of transmembrane voltage (MSORTV) to patterned growth cultures of heart cells to analyze the effect of geometry per se on impulse propagation in excitable tissue, with cellular and subcellular resolution. Extensive dye screening led to the choice of di-8-ANEPPS as the most suitable voltage-sensitive dye for this application; it is internalized slowly and permits optical recording with signal-to-noise ratios as high as 40:1 (measured peak-to-peak) and average fractional fluorescence changes of 15% per 100 mV. Using a x 100 objective and a fast data acquisition system, we could resolve impulse propagation on a microscopic scale (15 microns) with high temporal resolution (uncertainty of +/- 5 microseconds). We could observe the decrease in conduction velocity of an impulse propagating along a narrow cell strand as it enters a region of abrupt expansion, and we could explain this phenomenon in terms of the micro-architecture of the tissue. In contrast with the elongated and aligned cells forming the narrow strands, the cells forming the expansions were aligned at random and presented 2.5 times as many cell-to-cell appositions per unit length. If the decrease in conduction velocity results entirely from this increased number of cell-to-cell boundaries per unit length, the mean activation delay introduced by each boundary can be estimated to be 70 microseconds. Using this novel experimental system, we could also demonstrate the electrical coupling of fibroblasts and endotheloid cells to myocytes in culture.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugada J., Boersma L., Kirchhof C. J., Heynen V. V., Allessie M. A. Reentrant excitation around a fixed obstacle in uniform anisotropic ventricular myocardium. Circulation. 1991 Sep;84(3):1296–1306. doi: 10.1161/01.cir.84.3.1296. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S., Morad M. A new laser scanning system for measuring action potential propagation in the heart. Science. 1981 Oct 23;214(4519):453–456. doi: 10.1126/science.6974891. [DOI] [PubMed] [Google Scholar]
- Durrer D., van Dam R. T., Freud G. E., Janse M. J., Meijler F. L., Arzbaecher R. C. Total excitation of the isolated human heart. Circulation. 1970 Jun;41(6):899–912. doi: 10.1161/01.cir.41.6.899. [DOI] [PubMed] [Google Scholar]
- Fast V. G., Kléber A. G. Microscopic conduction in cultured strands of neonatal rat heart cells measured with voltage-sensitive dyes. Circ Res. 1993 Nov;73(5):914–925. doi: 10.1161/01.res.73.5.914. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Frostig R. D., Lieke E., Hildesheim R. Optical imaging of neuronal activity. Physiol Rev. 1988 Oct;68(4):1285–1366. doi: 10.1152/physrev.1988.68.4.1285. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Hildesheim R., Farber I. C., Anglister L. Improved fluorescent probes for the measurement of rapid changes in membrane potential. Biophys J. 1982 Sep;39(3):301–308. doi: 10.1016/S0006-3495(82)84520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez C. S., Plonsey R. Effect of resistive discontinuities on waveshape and velocity in a single cardiac fibre. Med Biol Eng Comput. 1987 Jul;25(4):428–438. doi: 10.1007/BF02443364. [DOI] [PubMed] [Google Scholar]
- Hyde A., Blondel B., Matter A., Cheneval J. P., Filloux B., Girardier L. Homo- and heterocellular junctions in cell cultures: an electrophysiological and morphological study. Prog Brain Res. 1969;31:283–311. doi: 10.1016/S0079-6123(08)63247-1. [DOI] [PubMed] [Google Scholar]
- Israel D. A., Edell D. J., Mark R. G. Time delays in propagation of cardiac action potential. Am J Physiol. 1990 Jun;258(6 Pt 2):H1906–H1917. doi: 10.1152/ajpheart.1990.258.6.H1906. [DOI] [PubMed] [Google Scholar]
- Jongsma H. J., van Rijn H. E. Electronic spread of current in monolayer cultures of neonatal rat heart cells. J Membr Biol. 1972;9(4):341–360. [PubMed] [Google Scholar]
- Kamino K. Optical approaches to ontogeny of electrical activity and related functional organization during early heart development. Physiol Rev. 1991 Jan;71(1):53–91. doi: 10.1152/physrev.1991.71.1.53. [DOI] [PubMed] [Google Scholar]
- Morad M., Salama G. Optical probes of membrane potential in heart muscle. J Physiol. 1979 Jul;292:267–295. doi: 10.1113/jphysiol.1979.sp012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey C. R., Clark J. W., Giles W. R. A model of slow conduction in bullfrog atrial trabeculae. Math Biosci. 1991 Sep;106(1):85–109. doi: 10.1016/0025-5564(91)90040-p. [DOI] [PubMed] [Google Scholar]
- Murphey C. R., Clark J. W., Giles W. R., Rasmusson R. L., Halter J. A., Hicks K., Hoyt B. Conduction in bullfrog atrial strands: simulations of the role of disc and extracellular resistance. Math Biosci. 1991 Sep;106(1):39–84. doi: 10.1016/0025-5564(91)90039-l. [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Pooler J. P., Narahashi T. Internal and external application of photodynamic sensitizers on squid giant axons. J Membr Biol. 1977 Sep 14;36(2-3):159–173. doi: 10.1007/BF01868149. [DOI] [PubMed] [Google Scholar]
- Parsons T. D., Salzberg B. M., Obaid A. L., Raccuia-Behling F., Kleinfeld D. Long-term optical recording of patterns of electrical activity in ensembles of cultured Aplysia neurons. J Neurophysiol. 1991 Jul;66(1):316–333. doi: 10.1152/jn.1991.66.1.316. [DOI] [PubMed] [Google Scholar]
- Pertsov A. M., Davidenko J. M., Salomonsz R., Baxter W. T., Jalife J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ Res. 1993 Mar;72(3):631–650. doi: 10.1161/01.res.72.3.631. [DOI] [PubMed] [Google Scholar]
- Pollard A. E., Burgess M. J., Spitzer K. W. Computer simulations of three-dimensional propagation in ventricular myocardium. Effects of intramural fiber rotation and inhomogeneous conductivity on epicardial activation. Circ Res. 1993 Apr;72(4):744–756. doi: 10.1161/01.res.72.4.744. [DOI] [PubMed] [Google Scholar]
- Robinson R. B., Legato M. J. Maintained differentiation in rat cardiac monolayer cultures: tetrodotoxin sensitivity and ultrastructure. J Mol Cell Cardiol. 1980 May;12(5):493–498. doi: 10.1016/0022-2828(80)90005-x. [DOI] [PubMed] [Google Scholar]
- Rohr S., Schölly D. M., Kléber A. G. Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res. 1991 Jan;68(1):114–130. doi: 10.1161/01.res.68.1.114. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Rudy Y., Quan W. L. A model study of the effects of the discrete cellular structure on electrical propagation in cardiac tissue. Circ Res. 1987 Dec;61(6):815–823. doi: 10.1161/01.res.61.6.815. [DOI] [PubMed] [Google Scholar]
- Salama G., Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science. 1976 Feb 6;191(4226):485–487. doi: 10.1126/science.191.4226.485. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Senseman D. M., Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983 Nov 3;306(5938):36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]
- Sawanobori T., Hirota A., Fujii S., Kamino K. Optical recording of conducted action potential in heart muscle using a voltage-sensitive dye. Jpn J Physiol. 1981;31(3):369–380. doi: 10.2170/jjphysiol.31.369. [DOI] [PubMed] [Google Scholar]
- Shumaker J. M., Clark J. W., Giles W. R. Simulations of passive properties and action potential conduction in an idealized bullfrog atrial trabeculum. Math Biosci. 1993 Aug;116(2):127–167. doi: 10.1016/0025-5564(93)90064-h. [DOI] [PubMed] [Google Scholar]
- Ursell P. C., Gardner P. I., Albala A., Fenoglio J. J., Jr, Wit A. L. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985 Mar;56(3):436–451. doi: 10.1161/01.res.56.3.436. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Allessie M. A., Bonke F. I., Lammers W., Smeets J., Fenoglio J. J., Jr Electrophysiologic mapping to determine the mechanism of experimental ventricular tachycardia initiated by premature impulses. Experimental approach and initial results demonstrating reentrant excitation. Am J Cardiol. 1982 Jan;49(1):166–185. doi: 10.1016/0002-9149(82)90292-2. [DOI] [PubMed] [Google Scholar]