Abstract
Background
Acute Achilles tendon rupture (AATR) surgical repair debates center on the clinical efficacy of minimally invasive surgery (MIS) versus open surgery (OS), with immobilization duration poorly stratified. This prospective cohort study aimed to compare clinical outcomes of OS and MIS for AATR repair and evaluate the impact of immobilization duration (0, 2, or 4 weeks) on postoperative rehabilitation.
Methods
A total of 474 patients undergoing surgical repair for acute AATR were stratified into six groups based on surgical approach (OS: 265 cases; MIS: 209 cases) and immobilization duration (0, 2, or 4 weeks). The primary outcomes were postoperative complications, while secondary outcomes included recovery times for Achilles tendon function. Data regarding the operative times, incision lengths, the visual analog scale (VAS) score, the Achilles tendon Total Rupture Score (ATRS), and the American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Scale score, and the relative Achilles tendon resting angle (ATRA) were also collected.
Results
MIS groups demonstrated significantly shorter operative times (34.1–34.4 vs. 45.1–46.1 min, P < 0.001) and reduced incision lengths (2.2–2.4 vs. 4.5–4.7 cm, P < 0.001) compared to OS. Postoperative VAS scores were markedly lower in MIS cohorts during the first 2 weeks (P < 0.001), with pain resolution comparable across all groups by 8 weeks. Despite the superior early functional recovery in PF (9.1–33.4 vs. 14.6–38.9 days, P < 0.001), Group D and E exhibited higher re-injury rates compared to OS (P < 0.05), in which Group D also demonstrated higher re-operation rates (5.6% vs. 0, P = 0.038). Prolonged immobilization (4 weeks) delayed functional recovery in both cohorts (P < 0.001). While transient differences in AOFAS Ankle-Hindfoot Scale and ATRS scores were observed at intermediate time points, all groups achieved near-maximal functional scores by 48 weeks, with no significant between-group differences (P > 0.05). Relative ATRA exhibited no significant intergroup differences at 48 weeks postoperatively (P > 0.05).
Conclusion
MIS for acute Achilles tendon rupture achieves faster early recovery but carries higher re-injury risks, mitigated by 4-week immobilization. OS benefits from shorter (2-week) immobilization. Both approaches yield equivalent long-term functions, emphasizing the need for tailored protocols and refined MIS techniques to optimize outcomes.
Trial registration
Keywords: Achilles tendon rupture, Minimally invasive surgery, Open repair, Postoperative immobilization, Functional recovery
The Achilles tendon, formed by the distal extensions of the gastrocnemius and soleus muscles, attaches to the calcaneal tuberosity and is among the strongest tendons in the human body [1- 3]. In recent years, acute Achilles tendon rupture (AATR) has emerged as a prevalent sports injury, particularly affecting active individuals and middle-aged men, as awareness of physical fitness increases [4- 7]. AATR results in pain and restricted plantar flexion, and delayed treatment may lead to tendon retraction and degeneration, adversely affecting healing and functional recovery [8- 10]. Compared to nonsurgical approaches, early surgical intervention is generally recommended for young, high-demand patients, as it effectively restores tendon continuity and facilitates accelerated rehabilitation [4, 9, 11- 13]. Open surgery (OS) allows direct visualization for tendon repair but is associated with larger incisions [14- 16]. Advances in minimally invasive surgery (MIS), such as the Achillon system, have introduced alternative approaches that minimize surgical trauma while effectively repairing the tendon [14, 16, 17].
Postoperative immobilization duration represents a central debate in rehabilitation strategies [18-20]. Early mobilization is advocated to accelerate functional recovery, yet insufficient immobilization may increase re-injury risks [18, 20]. Optimizing postoperative protocols to balance functional recovery with tendon protection remains a clinical challenge.
This study evaluates the impact of surgical approach (OS vs. MIS) and immobilization duration (0, 2, or 4 weeks) on clinical outcomes, aiming to refine evidence-based treatment strategies for acute Achilles tendon rupture (AATR).
Method
The study was reviewed and approved by our institutional review board (Peking University Third Hospital Medical Science Research Ethics Committee; IRB00006761- M2020315) and registered at ClinicalTrials. gov (NCT04663542).
Design and population
This prospective cohort study consecutively enrolled 512 patients who underwent surgical repair for acute Achilles tendon rupture at our institution from September 2022 to February 2024. All patients recruited before April 2023 received OS. Beginning in April 2023, a protocol modification mandated that all enrolled patients initially undergo MIS; conversion to OS was performed intraoperatively if anatomical contraindications precluded safe MIS completion. Exclusion criteria included excessive interlayer separation (> 20 mm) between the gastrocnemius-derived outer layer and soleus-derived inner layer, inadequate tendon end approximation due to retraction or fragility, or technical failure to achieve secure fixation. Following exclusions of 20 patients lost to follow-up and 18 requiring intraoperative conversion to OS, 474 participants (92.6% retention rate) constituted the final analytical cohort. Written informed consent was obtained from all participants prior to study inclusion.
Participants were further categorized into six groups according to the surgical method (OS: 265 cases; MIS: 209 cases) and postoperative immobilization duration (0, 2, or 4 weeks): A (OS, 0 weeks, n = 88), B (OS, 2 weeks, n = 85), C (OS, 4 weeks, n = 92), D (MIS, 0 weeks, n = 71), E (MIS, 2 weeks, n = 72), and F (MIS, 4 weeks, n = 66). All participants adhered to a standardized rehabilitation protocol after the removal of external immobilization [2, 18, 21].
Preoperative diagnosis was confirmed via ultrasound, which assessed the rupture site, severity, and anatomical characteristics, including the gap distance of the rupture site (GDRS) and the distance from the rupture site to the tendon insertion (DRSTI). Inclusion criteria comprised: (1) age 18–60 years; (2) closed Achilles tendon injury; (3) acute complete rupture (≤ 7 days post-injury); (4) DRSTI > 3.0 cm. Exclusion criteria were: (1) partial rupture; (2) ATR at the tendon insertion point; (3) comorbidities that may affect functional test results (e.g., diabetes, neuropathy, autoimmune diseases); (4) loss to follow-up.
Surgical procedure
Patients were positioned prone and operated under spinal anesthesia with thigh-level tourniquet control. Bilateral Achilles tendon resting angle (ATRA) measurements (fibular long axis to fifth metatarsal headline) were recorded preoperatively [22].
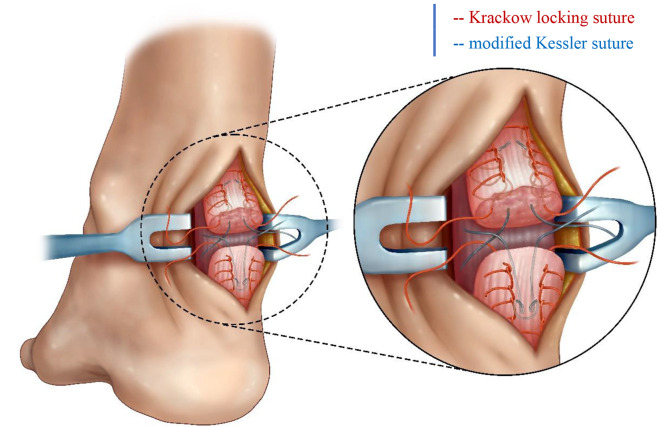
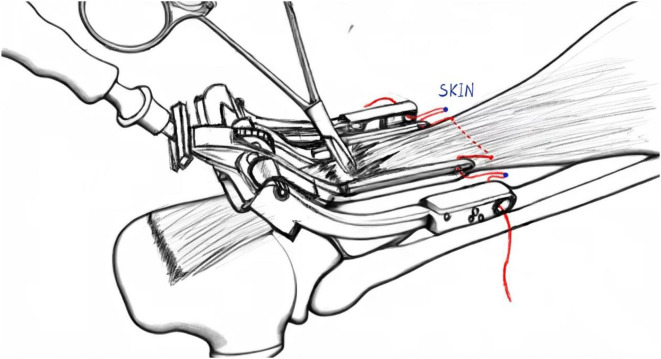
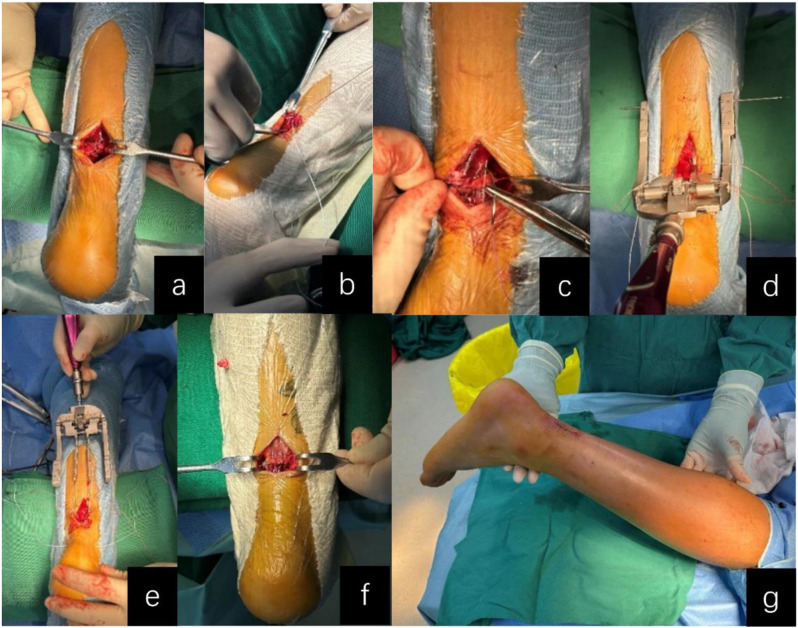
For minimally invasive surgery (MIS; Groups D-F), a 2–3 cm longitudinal incision was created at the rupture site, followed by careful blunt dissection of the paratenon to expose both proximal and distal tendon stumps. Irregular tendon ends were sharply debrided to facilitate optimal apposition. The tendon stumps were then grasped with Allis clamps to apply axial traction, allowing assessment of tendon tension and achievable elongation while minimizing adhesion formation through gentle dissection between the proximal tendon and paratenon using vascular forceps. The proximal stump was secured with two Krackow locking-loop sutures using No. 2 non-absorbable polyester sutures (ETHIBOND, Johnson & Johnson, USA), with suture trajectories depicted by red lines in Fig. 1. The dual medial prongs of the Achillon® instrument (Integra LifeSciences, USA) were carefully advanced through the potential space between the proximal tendon stump and paratenon. During insertion, the control nut was continuously rotated to facilitate gradual divergence of the medial prongs, ensuring optimal engagement with superior tendon fibers. Unlike conventional minimally invasive techniques that employ multiple percutaneous suture pairs with overlapping knots [14], our approach utilizes only a single suture pair to minimize potential nerve compression from multiple knot stacks. A guide needle loaded with absorbable No. 2 ETHIBOND suture (Johnson & Johnson, USA) was then passed sequentially through: (1) medial cutaneous tissue, (2) lateral prong of the Achillon device, (3) both medial prongs, (4) lateral cutaneous tissue, and (5) contralateral lateral prong. This trajectory guaranteed intratendinous suture placement while avoiding neurovascular structures. Following device withdrawal, the suture was externalized from the paratenon and tensioned to verify unrestricted gliding and adequate tendon purchase, creating a modified Kessler-type configuration (the red suture in Fig. 2). The distal tendon was addressed using identical instrumentation principles. Sutures were tensioned sequentially: Krackow sutures were tied first to establish primary stability, followed by the Achillon sutures to ensure anatomical alignment under intraoperative tension matching the contralateral ATRA. Dorsal and lateral reinforcement was achieved with four figure-of-eight sutures, with optional ventral sutures added if intraoperative stress testing demonstrated residual laxity. Finally, the paratenon was closed with 2 − 0 absorbable sutures (Monocryl™, Ethicon) and the skin with 4 − 0 absorbable sutures, followed by application of a below-knee brace in 30° plantar flexion. See Fig. 3 for details.
Fig. 1.
Krackow locking suture combined with modified Kessler suture
Fig. 2.
Achillon device withdrawal during minimally invasive Achilles tendon repair, demonstrating decompression suture (red line) traversing the surgical incision. Blue markers denote cutaneous penetration sites of the suture trajectory
Fig. 3.
Intraoperative Images of Minimally Invasive Achilles Tendon Repair: (a) Longitudinal incision exposure; (b) Tendon stump tension assessment; (c) Krackow locking suture configuration; (d) Decompression suture insertion via Achillon device; (e) Achillon device withdrawal; (f) Sequential suture tensioning; (g) Surgical incision closure
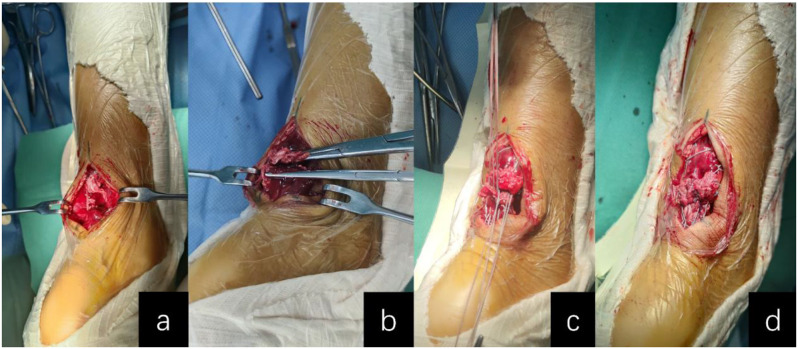
For open surgery (OS; Groups A-C), a traditional open repair was performed using the Krackow locking loop technique combined with a modified Kessler suture, as our previous study described [19]. The Krackow suture technique in the open surgery groups mirrored that used in the MIS groups, while the modified Kessler suture - like in the MIS approach - employed only a single suture pair (as illustrated in Fig. 1). See Fig. 4 for details.
Fig. 4.
Intraoperative Images of Open Achilles Tendon Repair: (a) Midline incision exposure of tendon stumps; (b) Debridement and fiber realignment of tendon ends; (c) Combined Krackow locking and modified Kessler sutures; (d) Sequential suture tensioning
All surgeries were conducted by the same team of surgeons following a standardized procedure.
Postoperative management
Groups A and D did not receive postoperative immobilization. Groups B and E received postoperative immobilization for two weeks, while Groups C and F underwent immobilization for four weeks. After the removal of the braces, all six groups began postoperative rehabilitation exercises according to the prescribed protocol (Table 1) [18]. During the early rehabilitation phase, the use of crutches was recommended to assist in training and to reduce the weight-bearing load on the affected limb.
Table 1.
Rehabilitation protocol
| After surgery | Postoperative exercise and immobilization with the brace for the corresponding time. |
|---|---|
| Immediately after removing the brace | Ankle mobilization |
| 0–2 weeks after removing the brace | Standing up for 1 h per day |
| 2–4 weeks after removing the brace |
Standing up for 2 h per day Deep squat |
| 4–6 weeks after removing the brace |
Double-legged heel raises Walking less than 1000 steps on flat ground |
| 6–8 weeks after removing the brace |
Single-legged heel raises Walking less than 2000 steps on flat ground |
| 2 weeks after successfully performing single-leg heel raises | Jogging |
| 4 weeks after successfully performing jogging | More vigorous training |
Data collection
Operative duration and wound length were recorded after surgery. Patients were assessed postoperatively at 1, 2 weeks, and 4, 8, 12, 24, and 48 weeks. Follow-up evaluations were standardized and performed by a single surgeon to ensure consistency. Clinical outcomes included the visual analog scale (VAS) for pain, the American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Scale [23], and the Achilles tendon Total Rupture Score (ATRS) [24], and the relative ATRA. VAS was utilized to assess pain intensity, employing a 10-cm linear scale ranging from “no pain” (0 cm, left endpoint) to “worst imaginable pain” (10 cm, right endpoint). Patients were instructed to mark their current pain level on the scale, with the corresponding value recorded as their VAS score. ATRS, a patient-reported outcome measure, consists of 10 items assessing symptom severity and physical activity limitations, with responses graded on an 11-point Likert scale (0–10 per item, total score range: 0-100), where higher scores indicate superior functional recovery. AOFAS Ankle-Hindfoot Scale, a clinician-administered tool, provides a comprehensive evaluation of ankle-hindfoot function with a maximum score of 100 points, categorized as excellent (90–100), good (75–89), fair (50–74), or poor (< 50). The relative ATRA is calculated by subtracting the non-injured side’s angle from the injured side’s angle, quantifying tendon length changes post-injury and during rehabilitation. A positive relative ATRA indicates the injured Achilles tendon has a larger resting angle than the non-injured side, suggesting shorter tendon length. Conversely, a negative value implies the injured tendon is longer, often due to elongation postoperation or insufficient healing.
Postoperative complications—including superficial infection, deep vein thrombosis (DVT), sural nerve palsy, Achilles tendon re-injury (confirmed by MRI following sudden falls), and re-operation (indicated for MRI-confirmed re-ruptures involving > 50% tendon width)—were meticulously documented. Functional recovery milestones were meticulously documented, including the time required to achieve the following outcomes: (1) restoration of plantarflexion (PF) to levels equivalent to the contralateral side; (2) resumption of light exercise (LE), defined as brisk walking and jogging; (3) return to work (RTW); and (4) recovery of one-leg heel-rise height (OHRH). The heel-rise height was measured as the vertical distance from the ground to the heel while the patient performed a heel rise with the knee fully extended (0° flexion) and without hyperextension. For standardized measurement, participants were instructed to slowly elevate the heel to maximal achievable height while maintaining full knee extension in the weight-bearing limb; rapid or compensatory movements were prohibited. Recovery time for OHRH was determined when the heel-rise height index (HRHI = [involved/uninvolved] × 100) reached 50% [25].
The primary outcome of the study was postoperative re-injury, while secondary outcomes included PF recovery times.
Statistical analyses
Statistical analyses were conducted using SPSS version 27.0 (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test was applied to evaluate the normality of the data distribution. Continuous variables exhibiting a normal distribution were expressed as mean ± standard deviation (SD). For stratified comparisons between OS and MIS groups, independent samples t-tests were employed. Within-group comparisons for both OS and MIS were conducted using one-way analysis of variance (ANOVA), with post-hoc pairwise comparisons performed using the Bonferroni correction to adjust for multiple comparisons.
Categorical variables, including gender, affected side, and the presence of complications, were analyzed using the χ² test. For datasets with expected frequencies < 1 or sample sizes < 40, Fisher’s exact test was employed to ensure accuracy. A p-value of < 0.05 was considered indicative of statistical significance.
Results
Baseline characteristics
A total of 474 patients were stratified into six groups: OS with 0-, 2-, or 4-week immobilization (A: n = 88; B: n = 85; C: n = 92) and MIS with corresponding immobilization durations (D: n = 71; E: n = 72; F: n = 66). Baseline characteristics, including sex distribution, age, affected side, BMI, GDRS, and DRSTI, showed no significant intergroup differences (all P > 0.05). However, MIS groups exhibited significantly shorter operation times (all P < 0.001) and reduced incision lengths (all P < 0.001) compared to OS groups (Table 2).
Table 2.
Baseline characteristics
| OS | MIS | P value | ||||
|---|---|---|---|---|---|---|
| P value | P value | |||||
| Gender (Male/Female) | 0w | 85/3 | 0.999 | 67/4 | 0.954 | 0.713 |
| 2w | 82/3 | 68/4 | 0.706 | |||
| 4w | 89/3 | 63/3 | 1.000 | |||
| Age (years) | 0w | 45.5 ± 14.7 | 0.667 | 42.6 ± 15.5 | 0.671 | 0.228 |
| 2w | 44.3 ± 15.4 | 44.4 ± 14.7 | 0.973 | |||
| 4w | 46.2 ± 11.6 | 44.5 ± 12.3 | 0.395 | |||
| Affected side (Right/Left) | 0w | 41/47 | 0.996 | 32/39 | 0.897 | 0.853 |
| 2w | 39/46 | 35/37 | 0.734 | |||
| 4w | 43/49 | 30/36 | 0.874 | |||
| Body Mass Index | 0w | 26.4 ± 1.6 | 0.056 | 26.0 ± 1.7 | 0.569 | 0.086 |
| 2w | 25.9 ± 1.7 | 26.2 ± 1.5 | 0.234 | |||
| 4w | 26.5 ± 2.3 | 26.3 ± 2.0 | 0.463 | |||
| Operation Time (min) | 0w | 46.1 ± 4.8 | 0.304 | 34.1 ± 4.6 | 0.913 | < 0.001 |
| 2w | 45.1 ± 3.5 | 34.4 ± 4.0 | < 0.001 | |||
| 4w | 45.4 ± 3.7 | 34.1 ± 3.1 | < 0.001 | |||
| GDRS (cm) | 0w | 1.7 ± 0.7 | 0.149 | 1.9 ± 0.6 | 0.820 | 0.054 |
| 2w | 1.9 ± 0.7 | 1.8 ± 0.6 | 0.689 | |||
| 4w | 1.9 ± 0.8 | 1.8 ± 0.9 | 0.735 | |||
| DRSTI (cm) | 0w | 4.3 ± 0.7 | 0.415 | 4.5 ± 0.8 | 0.060 | 0.233 |
| 2w | 4.2 ± 0.7 | 4.2 ± 0.6 | 0.837 | |||
| 4w | 4.3 ± 0.6 | 4.3 ± 0.7 | 0.701 | |||
| Incision length (cm) | 0w | 4.7 ± 0.8 | 0.347 | 2.3 ± 0.4 | 0.109 | < 0.001 |
| 2w | 4.5 ± 1.1 | 2.4 ± 0.4 | < 0.001 | |||
| 4w | 4.5 ± 1.0 | 2.2 ± 0.4 | < 0.001 | |||
Data represent the mean ± SD;
GDRS, gap distance of the rupture site; DRSTI, distance from the rupture site to the Achilles tendon insertion
Pain scores
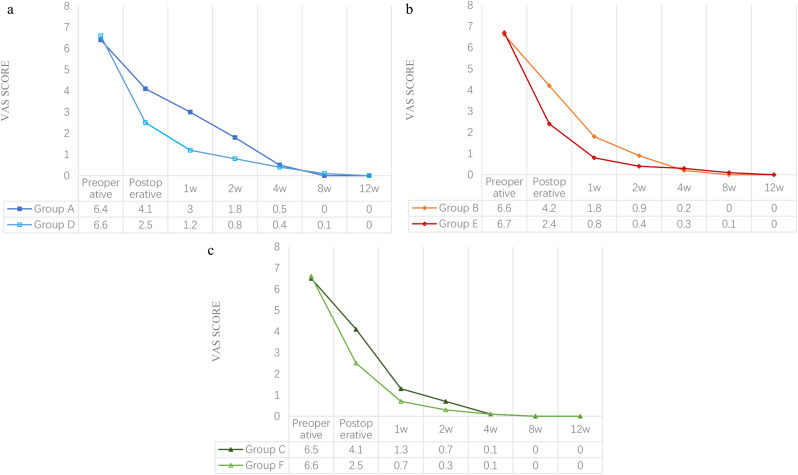
The VAS score for pain significantly decreased after surgery and reached 0 in all groups after 12 weeks. Preoperative pain scores were similar among the six groups (P > 0.05). Immediately postoperatively, VAS scores were significantly lower in MIS groups compared to OS groups (A vs. D = 4.1 ± 0.6 vs. 2.5 ± 0.6, B vs. E = 4.2 ± 0.6 vs. 2.4 ± 0.5, C vs. F = 4.1 ± 0.7 vs. 2.5 ± 0.8, P < 0.001), a trend that persisted at 1 week (A vs. D = 3.0 ± 0.8 vs. 1.2 ± 0.5, B vs. E = 1.8 ± 1.0 vs. 0.8 ± 0.5, C vs. F = 1.3 ± 0.8 vs. 0.7 ± 0.6, P < 0.001) and 2 weeks (A vs. D = 1.8 ± 0.7 vs. 0.8 ± 0.6, B vs. E = 0.9 ± 0.8 vs. 0.4 ± 0.5, C vs. F = 0.7 ± 0.6 vs. 0.3 ± 0.5, P < 0.001). Notably, the within-group analysis revealed higher VAS scores in Group A vs. Groups B and C (A vs. B vs. C = 3.0 ± 0.8 vs. 1.8 ± 1.0 vs. 1.3 ± 0.8 at 1 week; and 1.8 ± 0.7 vs. 0.9 ± 0.8 vs. 0.7 ± 0.6 at 2 weeks, both P < 0.001) and Group D vs. Groups E and F (D vs. E vs. F = 1.2 ± 0.5 vs. 0.8 ± 0.5 vs. 0.7 ± 0.6 at 1 week; and 0.8 ± 0.6 vs. 0.4 ± 0.5 vs. 0.3 ± 0.5 at 2 weeks, both P < 0.001) at 1 and 2 weeks. From week 4 onwards, there is no significant difference between OS and MIS groups (P > 0.05) (Fig. 5).
Fig. 5.
Visual Analogue Scale (VAS) for Pain: (a) Group A vs. Group D; (b) Group B vs. Group E; (c) Group C vs. Group F
Functional outcomes
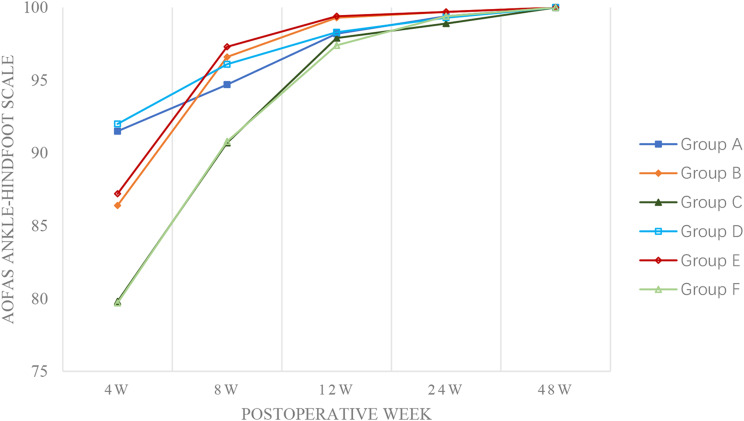
AOFAS Ankle-Hindfoot Scale and ATRS showed improvement over time in all groups. For the AOFAS Ankle-Hindfoot Scale, significant differences were only observed at specific intervals: Group D had higher scores than its counterpart at 8 weeks (96.1 ± 2.7 vs. 94.7 ± 1.9, P < 0.001) and Group F at 24 weeks (99.4 ± 0.8 vs. 98.9 ± 0.8, P < 0.001). Within-group comparisons revealed that Groups A and D had higher AOFAS Ankle-Hindfoot Scale scores at 4 weeks postoperatively (both P < 0.001), whereas Groups B and E maintained higher scores at 8, 12, and 24 months (all P < 0.05). By 48 weeks, AOFAS Ankle-Hindfoot Scale scores for all groups approached 100 with no significant between-group differences (Fig. 6).
Fig. 6.
American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Scale
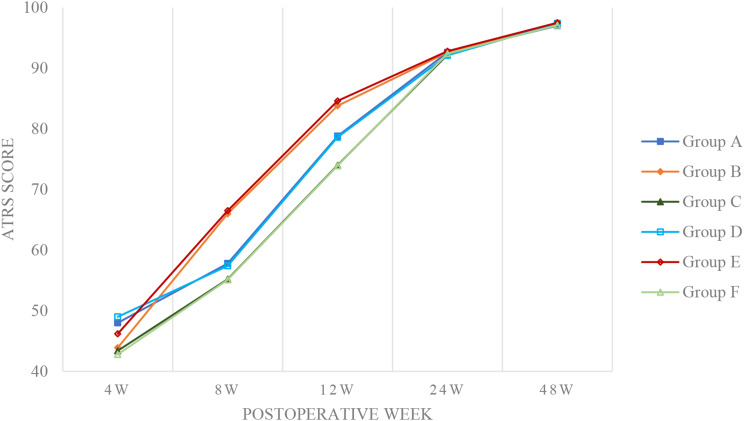
Similarly, for ATRS scores, notable differences were limited to certain periods: Group D scored higher at 4 weeks (49.0 ± 2.0 vs. 48.0 ± 1.9, P = 0.003) and Group E also showed higher scores at the same interval (46.2 ± 3.1 vs. 43.9 ± 2.7, P < 0.001). Within-group comparisons indicated that Groups A and D had higher ATRS scores at 4 weeks (both P < 0.001), while Groups B and E showed superiority at 8 and 12 weeks (all P < 0.001). No significant between-group differences in ATRS scores were noted at 24 and 48 weeks (Fig. 7).
Fig. 7.
Achilles tendon total rupture score (ATRS)
No significant differences in relative ATRA levels were observed among the six groups during the intraoperative period or at 48 weeks postoperatively (P > 0.05) (Table 3).
Table 3.
Relative ATRA
| Time | OS | MIS | P value | |||
|---|---|---|---|---|---|---|
| P value | P value | |||||
| Intraoperative (degrees) | 0w | 7.2 ± 1.7 | 0.096 | 7.3 ± 2.1 | 0.398 | 0.794 |
| 2w | 7.4 ± 1.9 | 7.3 ± 1.6 | 0.666 | |||
| 4w | 6.8 ± 2.2 | 6.9 ± 1.3 | 0.747 | |||
| 48w (degrees) | 0w | -5.2 ± 1.8 | 0.287 | -5.1 ± 1.7 | 0.857 | 0.920 |
| 2w | -4.8 ± 1.6 | -5.0 ± 1.9 | 0.458 | |||
| 4w | -5.0 ± 2.0 | -5.1 ± 1.9 | 0.659 | |||
Data represent the mean ± SD;
ATRA, Achilles tendon resting angle
Recovery times
No significant differences in OHRH, LE, or RTW recovery times were found between corresponding MIS and OS groups (all P > 0.05). However, MIS groups achieved faster PF recovery (all P < 0.001). Prolonged immobilization (4w) in both OS and MIS subgroups delayed PF, OHRH, LE, and RTW recovery (all P < 0.001) (Table 4). Groups that received no post-operative immobilization (Groups A and D) demonstrated the best in PF recovery (both P < 0.001).
Table 4.
Recovery time
| Time | OS | MIS | P value | |||
|---|---|---|---|---|---|---|
| P value | P value | |||||
| PF (days) | 0w | 14.6 ± 2.9 | < 0.001 | 9.1 ± 2.6 | < 0.001 | < 0.001 |
| 2w | 26.9 ± 3.6 a | 20.9 ± 3.7 a | < 0.001 | |||
| 4w | 38.9 ± 4.6 ab | 33.4 ± 4.4 ab | < 0.001 | |||
| LE (weeks) | 0w | 16.9 ± 1.3 | < 0.001 | 17.1 ± 1.9 | < 0.001 | 0.353 |
| 2w | 17.3 ± 2.0 | 17.0 ± 1.8 | 0.354 | |||
| 4w | 19.8 ± 1.7 ab | 19.3 ± 2.4 ab | 0.088 | |||
| RTW (weeks) | 0w | 5.6 ± 1.2 | < 0.001 | 5.6 ± 0.7 | < 0.001 | 0.932 |
| 2w | 5.9 ± 1.2 | 5.8 ± 0.7 | 0.447 | |||
| 4w | 6.6 ± 0.9 ab | 6.6 ± 0.7 ab | 0.753 | |||
| OHRH (weeks) | 0w | 12.4 ± 1.2 | < 0.001 | 12.5 ± 1.8 | < 0.001 | 0.467 |
| 2w | 11.9 ± 0.8 a | 12.2 ± 1.2 | 0.065 | |||
| 4w | 14.9 ± 1.6 ab | 14.8 ± 2.0 ab | 0.726 | |||
Data represent the mean ± SD;
OHRH, one-leg heel-rise height; LE, light exercise; RTW, return to work; PF, plantarflexion;
a significantly different from 0w groups (multiple pairwise within-group post hoc comparison tests);
b significantly different from 2w groups (multiple pairwise within-group post hoc comparison tests)
Complications
There were no significant differences in superficial infection, deep vein thrombosis, or sural nerve palsy rates among all 6 groups (all P > 0.05). The re-injury rate was significantly higher in Groups D and E compared to Groups A and B (D: 14.1%vs A: 2.3%, P = 0.006; E: 12.5% vs. B: 1.2%, P = 0.006). In MIS groups, Group F demonstrated a lower re-injury rate compared to Groups D and E (F: 1.5%, P = 0.026), with no significant difference between Groups D and E. Postoperative reoperation rates for tendon rupture were higher in Group D than Group A (P = 0.038), with no significant differences among other groups (Table 5).
Table 5.
Complication
| OS | MIS | P value | ||||
|---|---|---|---|---|---|---|
| Cases (%) | P value | Cases (%) | P value | |||
| Superficial infection | 0w | 3(3,4%) | 0.619 | 4(5.6%) | 0.878 | 0.701 |
| 2w | 5(5.9%) | 4(5.6%) | 1.000 | |||
| 4w | 6(6.5%) | 5(7.6%) | 1.000 | |||
| Deep vein thrombosis | 0w | 1(1.1%) | 1.000 | 0(0) | 0.765 | 1.000 |
| 2w | 0(0) | 1(1.4%) | 0.459 | |||
| 4w | 1(1.1%) | 1(1.5%) | 1.000 | |||
| Sural nerve palsy | 0w | 2(2.3%) | 0.873 | 0(0) | 0.764 | 0.503 |
| 2w | 1(1.2%) | 1(1.4%) | 1.000 | |||
| 4w | 3(3.3) | 1(1.5%) | 0.641 | |||
| Re-injury | 0w | 2(2.3%) | 0.317 | 10(14.1%) | 0.026 | 0.006 |
| 2w | 1(1.2%) | 9(12.5%) | 0.006 | |||
| 4w | 0(0) | 1 (1.5%) ab | 0.418 | |||
| Re-operation | 0w | 0(0) | - | 4(5.6%) | 0.321 | 0.038 |
| 2w | 0(0) | 1(1.4%) | 0.459 | |||
| 4w | 0(0) | 1(1.5%) | 0.418 | |||
Data represent the mean ± SD;
a significantly different from 0w groups (using the χ² test or Fisher’s exact test);
b significantly different from 2w groups (using the χ² test or Fisher’s exact test)
Discussion
AATR predominantly occurs in physically active individuals, particularly during sports requiring explosive movements such as sprinting or jumping [2, 4, 18, 26, 27], where sudden acceleration or deceleration generates tensile forces exceeding 12.5 times body weight [28, 29]. Biomechanically, the midportion of the tendon, characterized by its hypovascularity and repetitive stress concentration, is the comparatively vulnerable site [1, 28, 30]. This region’s limited blood supply impairs self-repair capacity, predisposing it to cumulative microtrauma and eventual failure under high-load conditions [31]. Epidemiologically, AATR exhibits a striking male predominance (male-to-female ratio: 22.7:1 in our cohort), which aligns with global trends [4, 26, 32]. While this disparity may reflect higher male participation in high-risk sports, intrinsic biological factors such as sex-specific collagen composition, hormonal influences, and differences in tendon stiffness warrant further investigation [11, 33]. Notably, non-sport-related AATR cases, often associated with systemic risk factors (e.g., obesity, diabetes, corticosteroid use, or fluoroquinolone exposure), are increasingly reported, emphasizing the need to broaden research beyond athletic populations [24, 34, 35]. Current therapeutic strategies prioritize anatomical restoration and functional rehabilitation to mitigate complications like re-injury or prolonged immobilization-induced joint stiffness [18, 21, 36, 37]. However, debates persist regarding optimal surgical techniques and postoperative protocols [14-16, 38].
In this prospective cohort study, MIS cohorts exhibited significantly reduced incision lengths (P < 0.001) and operative times (P < 0.001) compared to OS, aligning with Grassi et al.’s meta-analysis of 358 patients (mean difference of surgical duration: -18.98 min; 95% CI: -11.14 to -26.82; P = 0.00001) [39]. The limited tissue dissection in MIS contributed to lower early postoperative VAS scores (P < 0.001 at first 2 weeks), which facilitated accelerated plantarflexion recovery (P < 0.001) by minimizing adhesion formation and preserving paratenon integrity [11, 38, 40]. Despite these early advantages, both approaches achieved comparable long-term functional restoration, with ATRS and AOFAS Ankle-Hindfoot Scale scores approaching 100 across all groups by 48 weeks (P > 0.05).
The Achillon instrument, a stainless-steel guide introduced by Assal et al. [41], facilitates MIS repair of AATR through a 2–3 cm longitudinal incision. Its dual-branch design with aligned apertures enables precise percutaneous suture deployment proximal and distal to the rupture site, preserving paratenon integrity and vascular supply while minimizing soft tissue trauma [41]. Conventional MIS protocols involved placing three sutures through distinct paired apertures in the device. However, Gatz et al.’s meta-analysis of 2,223 surgical cases (1,055 open vs. 1,168 MIS) [16] demonstrated a significantly higher risk of sural nerve injury in MIS cohorts compared to open repair (OS vs. MIS = 2.4% vs. 7.3%, OR: 0.45; 95% CI: 0.28–0.74; P = 0.001). This disparity may result from blind suturing and the use of multiple percutaneous sutures, which can inadvertently entrap or encircle nerve branches, thereby increasing the risk of direct nerve laceration or indirect irritation caused by perineural suture loops [5, 16]. To address the elevated risk of sural nerve injury [42], we modified the procedure by implementing a simplified suturing protocol: a single decompression suture is deployed through the paired guide apertures in each tendon stump. The percentage of sural nerve palsy in our research was 1.0% in the MIS group, which is much lower than in previous studies [15, 16, 39].
Meanwhile, many patients undergoing MIS in this study were young amateur athletes driven by rapid functional recovery goals. This eagerness, combined with a premature return to high-impact activities, such as single-leg hopping, predisposed them to accidental falls, resulting in higher rates of re-injury in Groups D and E (14.1% and 12.5%, respectively; P < 0.05) and re-operation rates in Group D (5.6% vs. 0 in Group A; P = 0.038). To mitigate this, our institution is prototyping a next-generation Achillon device featuring the deployment of multiple sutures through a single pair of guide apertures, thereby increasing suture density and enhancing biomechanical stability at the repair site.
The duration of postoperative immobilization significantly influenced recovery outcomes. Shorter immobilization periods (0 and 2 weeks) in both OS and MIS groups optimized functional recovery, particularly in PF recovery. Conversely, prolonged immobilization (4 weeks) delayed recovery metrics such as OHRH, LE, and RTW (all P < 0.001). Notably, the relative ATRA showed no statistically significant differences between surgical approaches (OS vs. MIS) or immobilization durations at 48 weeks (P > 0.05). This suggests that neither the repair technique nor immobilization protocol substantially influenced structural tendon lengthening, as ATRA indirectly reflects cumulative elongation through resting ankle positioning [43]. This finding is consistent with the prior study’s emphasis on standardized rehabilitation protocols, which advocate for early mobilization to enhance functional outcomes [14, 18, 21]. In the OS cohort, a 2-week immobilization protocol may represent the optimal strategy for early rehabilitation, as it was associated with relatively lower VAS pain scores, improved functional recovery, and no significant increase in complication rates, consistent with our prior study [18]. However, the higher re-injury and re-operation rates in MIS groups with shorter immobilization suggest a need for careful balance between early mobilization and tendon protection. The ATRA data further support this balance: despite equivalent long-term tendon length outcomes across groups, differential re-injury risks highlight the importance of protecting MIS repairs during early healing phases. These findings reinforce the rationale for 4-week immobilization protocols for MIS patients, particularly in high-demand populations.
This study has several limitations despite its clinical insights. First, preoperative activity levels and habitual loading patterns—critical confounders for rehabilitation—were not objectively quantified due to insufficient granularity in patient-reported exertion metrics, despite comparable occupational distributions. Second, the exclusion of 18 patients converted intraoperatively from MIS to OS introduced cohort heterogeneity, potentially limiting between-group comparability. Third, functional recovery milestones lacked objective biomechanical parameters (e.g., tendon lengthening or ROM) and relied on clinician-reported timelines rather than instrumented gait analysis or ultrasound-based quantification. Future studies should integrate imaging modalities to evaluate structural changes like tendon elongation alongside functional outcomes, complemented by large-scale RCTs with standardized protocols to validate findings. This work advances evidence for personalized AATR management by critically weighing the trade-offs between MIS and OS.
Conclusion
This study demonstrates that both minimally invasive surgery and open repair for acute Achilles tendon rupture yield excellent long-term functional outcomes. In this study MIS had a significantly higher re-injury and re-operation rate compared to open surgery. There were no significant differences in superficial infection rates, DVT or sural nerve palsy. MIS did offer better early pain resolution and faster plantar flexion recovery. Prolonged immobilization (4 weeks) for MIS groups may mitigate re-injury risks while balancing functional recovery. In contrast, a 2-week immobilization protocol may represent the optimal for OS groups. Future research should focus on refining MIS techniques, validating findings in larger cohorts, and exploring advanced rehabilitation strategies to enhance outcomes.
Acknowledgements
Thanks to the leaders and colleagues for their guidance and strong support in the process of scientific research and paper writing, and thank the patients who cooperated with the follow-up.
Author contributions
Yuan Cao and Xiuzhi Li contributed equally to this study. Yuan Cao and Xiuzhi Li: Data collection and analysis, article writing and revision; Yang Lv and Gao Si: Surgery, experimental design; Zengzhen Cui: Experimental design, research guidance, and supervision.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
First authors.
Contributor Information
Yang Lv, Email: lvyang42@126.com.
Gao Si, Email: si_gao@126.com.
References
- 1.Maganaris CN, Narici MV, Maffulli N. Biomechanics of the Achilles tendon. Disabil Rehabil. 2008;30:1542–7. [DOI] [PubMed] [Google Scholar]
- 2.Tarantino D, Palermi S, Sirico F, Corrado B. Achilles tendon rupture: mechanisms of injury, principles of rehabilitation and return to play. J Funct Morphol Kinesiol. 2020;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross CE, Nunley JA. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233–9. [DOI] [PubMed] [Google Scholar]
- 4.Sheth U, Wasserstein D, Jenkinson R, Moineddin R, Kreder H, Jaglal SB. The epidemiology and trends in management of acute Achilles tendon ruptures in ontario, canada: a population-based study of 27 607 patients. Bone Joint J. 2017;99–B:78–86. [DOI] [PubMed] [Google Scholar]
- 5.Melinte MA, Nistor DV, de Souza Conde RA, Hernández RG, Wijaya P, Marvin K, et al. Mini-open versus percutaneous surgical repair for acute Achilles tendon rupture: a systematic review and meta-analysis. Int Orthop. 2025;49:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maffulli N, Irwin AS, Kenward MG, Smith F, Porter RW. Achilles tendon rupture and sciatica: a possible correlation. Br J Sports Med. 1998;32:174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkateshaiah S, Reddy AGR, Srikantaiah VC. Transfer of the flexor hallucis longus tendon for neglected and degenerative tendo achillis rupture: A prospective clinical study. MLTJ-Muscles Ligaments Tendons J. 2023;13:243–51. [Google Scholar]
- 8.Deng S, Sun Z, Zhang C, Chen G, Li J. Surgical treatment versus Conservative management for acute Achilles tendon rupture: A systematic review and Meta-Analysis of randomized controlled trials. J Foot Ankle Surg. 2017;56:1236–43. [DOI] [PubMed] [Google Scholar]
- 9.Khan RJ, Carey Smith RL. Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev. 2010;CD003674. [DOI] [PubMed]
- 10.Bergamin F, Civera M, Reinoso MR, Burgio V, Ruiz OG, Surace C. Worldwide incidence and surgical costs of tendon injuries: A systematic review and Meta-Analysis. MLTJ-Muscles Ligaments Tendons J. 2023;13:31–45. [Google Scholar]
- 11.Willits K, Amendola A, Bryant D, Mohtadi NG, Giffin JR, Fowler P, et al. Operative versus nonoperative treatment of acute Achilles tendon ruptures: a multicenter randomized trial using accelerated functional rehabilitation. J Bone Joint Surg Am. 2010;92:2767–75. [DOI] [PubMed] [Google Scholar]
- 12.Meulenkamp B, Woolnough T, Cheng W, Shorr R, Stacey D, Richards M, et al. What is the best evidence to guide management of acute Achilles tendon ruptures?? A systematic review and network Meta-Analysis of randomized controlled trials. Clin Orthop Relat Res. 2021;479:2119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maffulli N. Current concepts in the management of subcutaneous tears of the Achilles tendon. Bull Hosp Jt Dis. 1998;57:152–8. [PubMed] [Google Scholar]
- 14.Fischer S, Colcuc C, Gramlich Y, Stein T, Abdulazim A, von Welck S, et al. Prospective randomized clinical trial of open operative, minimally invasive and Conservative treatments of acute Achilles tendon tear. Arch Orthop Trauma Surg. 2021;141:751–60. [DOI] [PubMed] [Google Scholar]
- 15.Attia AK, Mahmoud K, d’Hooghe P, Bariteau J, Labib SA, Myerson MS. Outcomes and complications of open versus minimally invasive repair of acute Achilles tendon ruptures: A systematic review and Meta-analysis of randomized controlled trials. Am J Sports Med. 2023;51:825–36. [DOI] [PubMed] [Google Scholar]
- 16.Gatz M, Driessen A, Eschweiler J, Tingart M, Migliorini F. Open versus minimally-invasive surgery for Achilles tendon rupture: a meta-analysis study. Arch Orthop Trauma Surg. 2021;141:383–401. [DOI] [PubMed] [Google Scholar]
- 17.Maffulli N, Christidis G, Gougoulias N, Christidis P, Poku D, Hassan R, et al. Percutaneous repair of the Achilles tendon with one knot offers equivalent results as the same procedure with two knots. A comparative prospective study. Br Med Bull. 2025;153:ldae019. [DOI] [PubMed] [Google Scholar]
- 18.Cao Y, Gao S, Cui Z, Fu Y, Bai L, Si G, et al. Comparison of different immobilisation durations following open surgery for acute Achilles tendon rupture: a prospective cohort study. J Orthop Surg Res. 2024;19:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu XY, Gao S, Lv Y, Zhou F, Jiao C, Fan JX, et al. Duration of immobilisation after Achilles tendon rupture repair by open surgery: a retrospective cohort study. J Orthop Surg Res. 2021;16:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormack R, Bovard J. Early functional rehabilitation or cast immobilisation for the postoperative management of acute Achilles tendon rupture? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2015;49:1329–35. [DOI] [PubMed] [Google Scholar]
- 21.Brumann M, Baumbach SF, Mutschler W, Polzer H. Accelerated rehabilitation following Achilles tendon repair after acute rupture - Development of an evidence-based treatment protocol. Injury. 2014;45:1782–90. [DOI] [PubMed] [Google Scholar]
- 22.Carmont MR, Grävare Silbernagel K, Brorsson A, Olsson N, Maffulli N, Karlsson J. The Achilles tendon resting angle as an indirect measure of Achilles tendon length following rupture, repair, and rehabilitation. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2015;2:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349–53. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson-Helander K, Thomeé R, Silbernagel KG, Thomeé P, Faxén E, Eriksson BI, et al. The Achilles tendon total rupture score (ATRS): development and validation. Am J Sports Med. 2007;35:421–6. [DOI] [PubMed] [Google Scholar]
- 25.Mansfield K, Dopke K, Koroneos Z, Bonaddio V, Adeyemo A, Aynardi M. Achilles tendon ruptures and repair in Athletes-a review of Sports-Related Achilles injuries and return to play. Curr Rev Musculoskelet Med. 2022;15:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briggs-Price S, Mangwani J, Houchen-Wolloff L, Modha G, Fitzpatrick E, Faizi M, et al. Incidence, demographics, characteristics and management of acute Achilles tendon rupture: an epidemiological study. PLoS ONE. 2024;19:e0304197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godoy-Santos AL, Bruschini H, Cury J, Srougi M, de Cesar-Netto C, Fonseca LF, et al. Fluoroquinolones and the risk of Achilles tendon disorders: update on a neglected complication. Urology. 2018;113:20–5. [DOI] [PubMed] [Google Scholar]
- 28.Komi PV. Relevance of in vivo force measurements to human biomechanics. J Biomech. 1990;23(Suppl 1):23–34. [DOI] [PubMed] [Google Scholar]
- 29.Gajhede-Knudsen M, Ekstrand J, Magnusson H, Maffulli N. Recurrence of Achilles tendon injuries in elite male football players is more common after early return to play: an 11-year follow-up of the UEFA champions league injury study. Br J Sports Med. 2013;47:763–8. [DOI] [PubMed] [Google Scholar]
- 30.Ilhami K, Gokhan M, Ulukan I, Eray BM, Levent A, Ciğdem T. Biomechanical and histologic comparison of Achilles tendon ruptures reinforced with intratendinous and peritendinous plantaris tendon grafts in rabbits: an experimental study. Arch Orthop Trauma Surg. 2004;124:608–13. [DOI] [PubMed] [Google Scholar]
- 31.Kasar ZS, Demirci B, Hunler H, Basaloglu H. The effect of L-Carnitine on experimental Achilles tendon injury. MLTJ-Muscles Ligaments Tendons J. 2023;13:524–30. [Google Scholar]
- 32.Gwynne-Jones DP, Sims M, Handcock D. Epidemiology and outcomes of acute Achilles tendon rupture with operative or nonoperative treatment using an identical functional bracing protocol. Foot Ankle Int. 2011;32:337–43. [DOI] [PubMed] [Google Scholar]
- 33.Soroceanu A, Sidhwa F, Aarabi S, Kaufman A, Glazebrook M. Surgical versus nonsurgical treatment of acute Achilles tendon rupture: a meta-analysis of randomized trials. J Bone Joint Surg Am. 2012;94:2136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suchak AA, Bostick GP, Beaupré LA, Durand DC, Jomha NM. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon. J Bone Joint Surg Am. 2008;90:1876–83. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen PV, Strange JE, Holt A. Oral fluoroquinolones and the risk of Achilles tendon rupture. J Sport Health Sci. 2024;13:749–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, Wang C, Ma X, Wang X, Zhang C, Chen L. Rehabilitation regimen after surgical treatment of acute Achilles tendon ruptures: a systematic review with meta-analysis. Am J Sports Med. 2015;43:1008–16. [DOI] [PubMed] [Google Scholar]
- 37.Guevara-Chavez FM, Caballero-Alvarado J, Zavaleta-Corvera C. Efficacy of surgical management versus Conservative management to decrease the incidence of Re-Rupture in adult patients with Achilles tendon rupture: A systematic review and Meta-Analysis. MLTJ-Muscles Ligaments Tendons J. 2024;14:54–66. [Google Scholar]
- 38.Marrone W, Andrews R, Reynolds A, Vignona P, Patel S, O’Malley M. Rehabilitation and return to sports after Achilles tendon repair. Int J Sports Phys Ther. 2024;19:1152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grassi A, Amendola A, Samuelsson K, Svantesson E, Romagnoli M, Bondi A, et al. Minimally invasive versus open repair for acute Achilles tendon rupture: Meta-Analysis showing reduced complications, with similar outcomes, after minimally invasive surgery. J Bone Joint Surg Am. 2018;100:1969–81. [DOI] [PubMed] [Google Scholar]
- 40.Healy C, Mulhall KJ, Nelligan M, Murray P, Bouchier-Hayes D. Postoperative stiffness and adhesion formation around repaired and immobilized Achilles tenotomies are prevented using a model of heat shock protein induction. J Surg Res. 2004;120:225–9. [DOI] [PubMed] [Google Scholar]
- 41.Assal M, Jung M, Stern R, Rippstein P, Delmi M, Hoffmeyer P. Limited open repair of Achilles tendon ruptures: a technique with a new instrument and findings of a prospective multicenter study. J Bone Joint Surg Am. 2002;84:161–70. [PubMed] [Google Scholar]
- 42.Xu L, Jin J, Liu Z, Wu M, Peng B, Jiang J, et al. A new technique of Achilles tendon rupture repaired by double transverse Mini-incision to avoid Sural nerve injury: A consecutive retrospective study. Orthop Surg. 2023;15:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zellers JA, Carmont MR, Silbernagel KG. Achilles tendon resting angle relates to tendon length and function. Foot Ankle Int. 2018;39:343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.