Abstract
The effects of static and low-frequency magnetic fields on gramicidin A channels have been investigated using bilayer patch clamp recording and a bridge technique capable of detecting 0.3% changes in the conductance of glyceryl monooleate membranes containing many channels. In the bridge technique the conductance was assessed using 10-ms voltage pulses applied at 10 Hz. Measurements were made for LiCl, KCl, and CsCl using magnetic fields of 50, 100, 500, and 5000 microT with the frequency scanned from 10-200 Hz. The combinations of static and low-frequency fields employed include the "cyclotron resonance" conditions at which effects had been predicted to occur. In no case was there any detectable change in conductance when the magnetic fields were applied or changed. Potassium currents through single gramicidin channels have been recorded for patches in which several channels may be open at once. Fields were applied for 2 min periods interleaved with 2 min controls. Methods have been developed to analyze the multichannel records to reveal the amplitude and duration of the channels together with the frequency, depth, and apparent period of flickers. No significant differences were observed between the control and field-exposed recording periods. The peak of the distribution of opening and closing transitions always coincided for fields on and off within the resolution, 0.4%, of the recordings. There are at least two types of flicker, one with typical period less than 0.1 ms, the other with typical period from 0.3-0.8 ms. Most of the latter were not complete closures with the conductance during a flicker 15-20% above the level for a full closure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair RK. Constraints on biological effects of weak extremely-low-frequency electromagnetic fields. Phys Rev A. 1991 Jan 15;43(2):1039–1048. doi: 10.1103/physreva.43.1039. [DOI] [PubMed] [Google Scholar]
- Adair RK. Reply to "Comment on 'Constraints on biological effects of weak extremely-low-frequency electromagnetic fields' ". Phys Rev A. 1992 Aug 15;46(4):2185–2187. doi: 10.1103/physreva.46.2185. [DOI] [PubMed] [Google Scholar]
- Adey W. R. Introduction: Effects of electromagnetic radiation on the nervous system. Ann N Y Acad Sci. 1975 Feb 28;247:15–20. doi: 10.1111/j.1749-6632.1975.tb35980.x. [DOI] [PubMed] [Google Scholar]
- Adey W. R. Tissue interactions with nonionizing electromagnetic fields. Physiol Rev. 1981 Apr;61(2):435–514. doi: 10.1152/physrev.1981.61.2.435. [DOI] [PubMed] [Google Scholar]
- Andersen O. S. Ion movement through gramicidin A channels. Single-channel measurements at very high potentials. Biophys J. 1983 Feb;41(2):119–133. doi: 10.1016/S0006-3495(83)84414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman C. F., Benane S. G., House D. E., Elliott D. J. Importance of alignment between local DC magnetic field and an oscillating magnetic field in responses of brain tissue in vitro and in vivo. Bioelectromagnetics. 1990;11(2):159–167. doi: 10.1002/bem.2250110207. [DOI] [PubMed] [Google Scholar]
- Blackman C. F., Benane S. G., Rabinowitz J. R., House D. E., Joines W. T. A role for the magnetic field in the radiation-induced efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics. 1985;6(4):327–337. doi: 10.1002/bem.2250060402. [DOI] [PubMed] [Google Scholar]
- Busath D., Szabo G. Gramicidin forms multi-state rectifying channels. Nature. 1981 Nov 26;294(5839):371–373. doi: 10.1038/294371a0. [DOI] [PubMed] [Google Scholar]
- Elliott J. R., Needham D., Dilger J. P., Haydon D. A. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim Biophys Acta. 1983 Oct 26;735(1):95–103. doi: 10.1016/0005-2736(83)90264-x. [DOI] [PubMed] [Google Scholar]
- Galt S., Sandblom J., Hamnerius Y., Höjevik P., Saalman E., Nordén B. Experimental search for combined AC and DC magnetic field effects on ion channels. Bioelectromagnetics. 1993;14(4):315–327. doi: 10.1002/bem.2250140404. [DOI] [PubMed] [Google Scholar]
- Galt S., Sandblom J., Hamnerius Y. Theoretical study of the resonant behaviour of an ion confined to a potential well in a combination of AC and DC magnetic fields. Bioelectromagnetics. 1993;14(4):299–314. doi: 10.1002/bem.2250140403. [DOI] [PubMed] [Google Scholar]
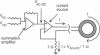
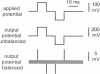
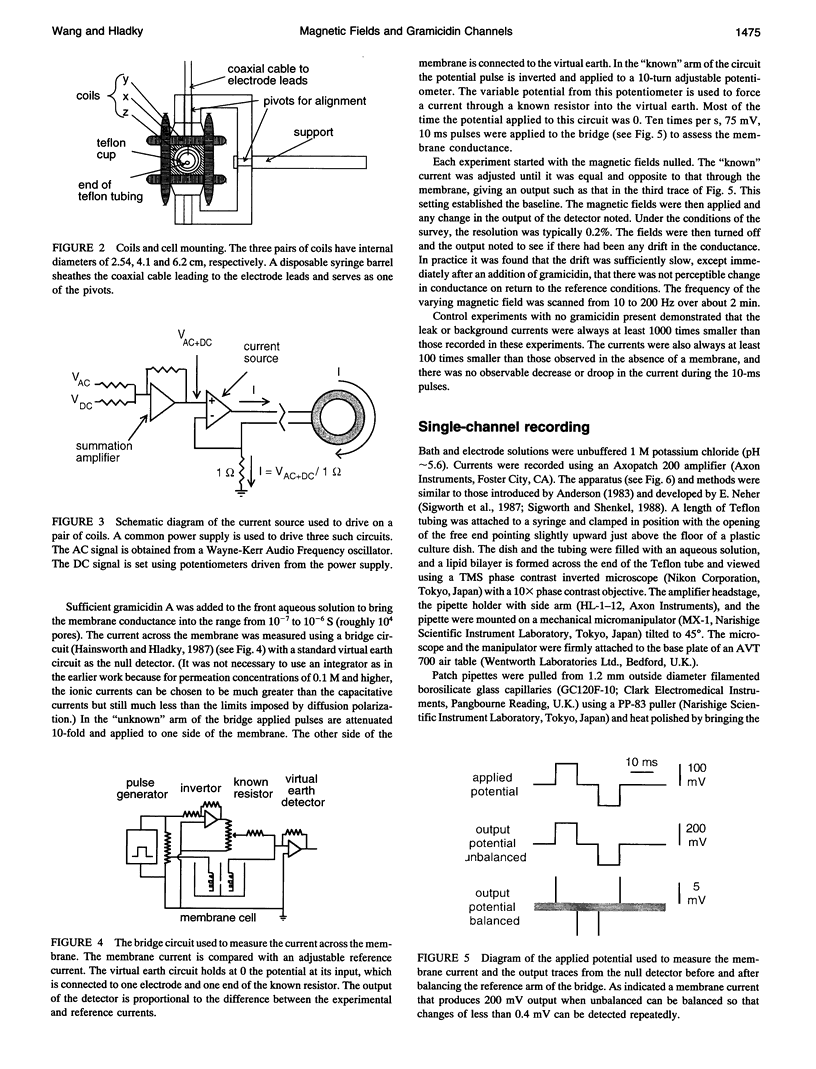
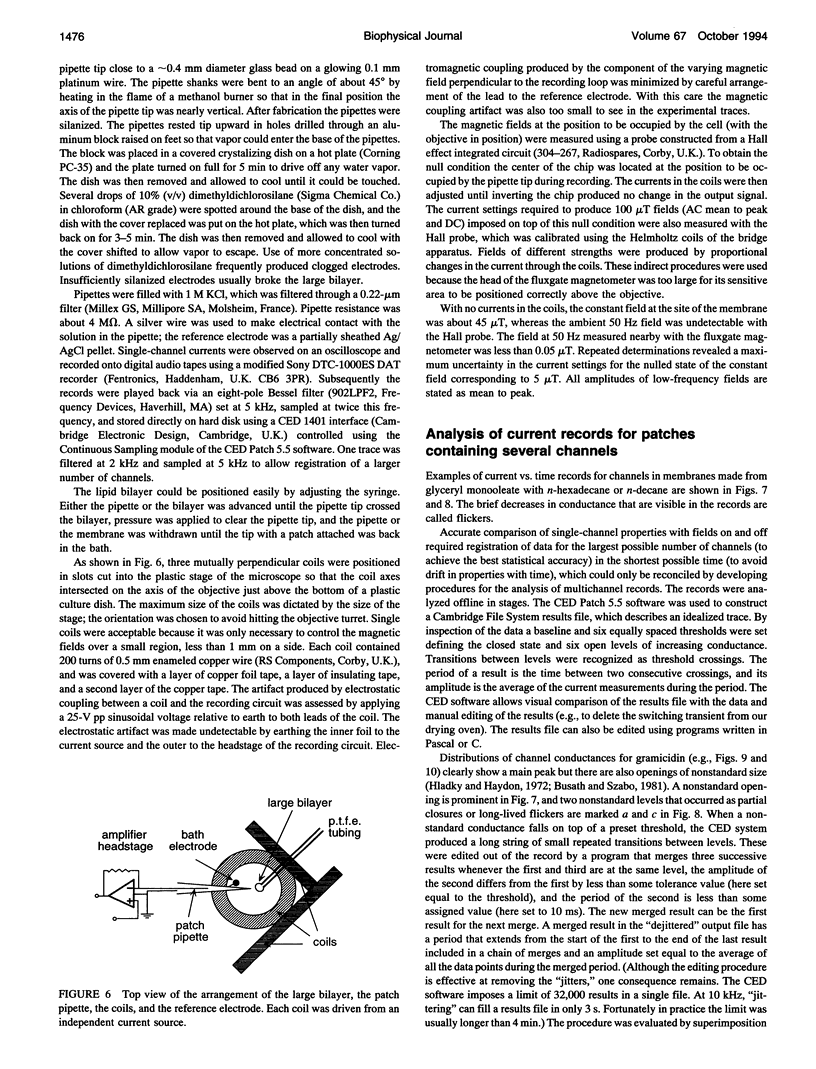
- Hainsworth A. H., Hladky S. B. Effects of double-layer polarization on ion transport. Biophys J. 1987 Jan;51(1):27–36. doi: 10.1016/S0006-3495(87)83308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle B. On the cyclotron resonance mechanism for magnetic field effects on transmembrane ion conductivity. Bioelectromagnetics. 1988;9(4):381–385. doi: 10.1002/bem.2250090408. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972 Aug 9;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Kirschvink JL. Comment on "Constraints on biological effects of weak extremely-low-frequency electromagnetic fields". Phys Rev A. 1992 Aug 15;46(4):2178–2184. doi: 10.1103/physreva.46.2178. [DOI] [PubMed] [Google Scholar]
- Liboff A. R., McLeod B. R. Kinetics of channelized membrane ions in magnetic fields. Bioelectromagnetics. 1988;9(1):39–51. doi: 10.1002/bem.2250090104. [DOI] [PubMed] [Google Scholar]
- Parkinson W. C., Sulik G. L. Diatom response to extremely low-frequency magnetic fields. Radiat Res. 1992 Jun;130(3):319–330. [PubMed] [Google Scholar]
- Reese J. A., Frazier M. E., Morris J. E., Buschbom R. L., Miller D. L. Evaluation of changes in diatom mobility after exposure to 16-Hz electromagnetic fields. Bioelectromagnetics. 1991;12(1):21–25. doi: 10.1002/bem.2250120104. [DOI] [PubMed] [Google Scholar]
- Ring A. Brief closures of gramicidin A channels in lipid bilayer membranes. Biochim Biophys Acta. 1986 Apr 25;856(3):646–653. doi: 10.1016/0005-2736(86)90160-4. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Urry D. W., Prasad K. U. Open channel noise. III. High-resolution recordings show rapid current fluctuations in gramicidin A and four chemical analogues. Biophys J. 1987 Dec;52(6):1055–1064. doi: 10.1016/S0006-3495(87)83299-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerra A., Brickmann J. Simulation of voltage-driven hydrated cation transport through narrow transmembrane channels. Biophys J. 1987 Jun;51(6):977–983. doi: 10.1016/S0006-3495(87)83425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. D., McLeod B. R., Liboff A. R., Cooksey K. Calcium cyclotron resonance and diatom mobility. Bioelectromagnetics. 1987;8(3):215–227. doi: 10.1002/bem.2250080302. [DOI] [PubMed] [Google Scholar]
- Wertheimer N., Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. 1979 Mar;109(3):273–284. doi: 10.1093/oxfordjournals.aje.a112681. [DOI] [PubMed] [Google Scholar]
- Woolley G. A., Wallace B. A. Model ion channels: gramicidin and alamethicin. J Membr Biol. 1992 Aug;129(2):109–136. doi: 10.1007/BF00219508. [DOI] [PubMed] [Google Scholar]