Abstract
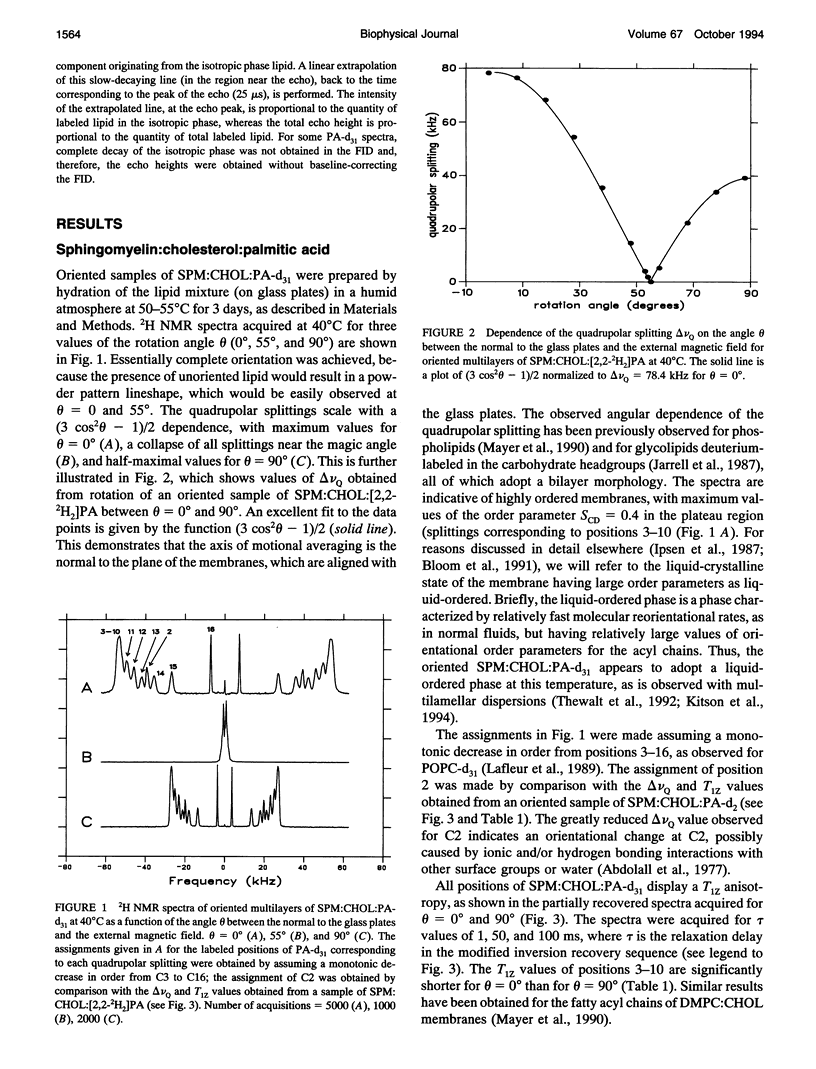
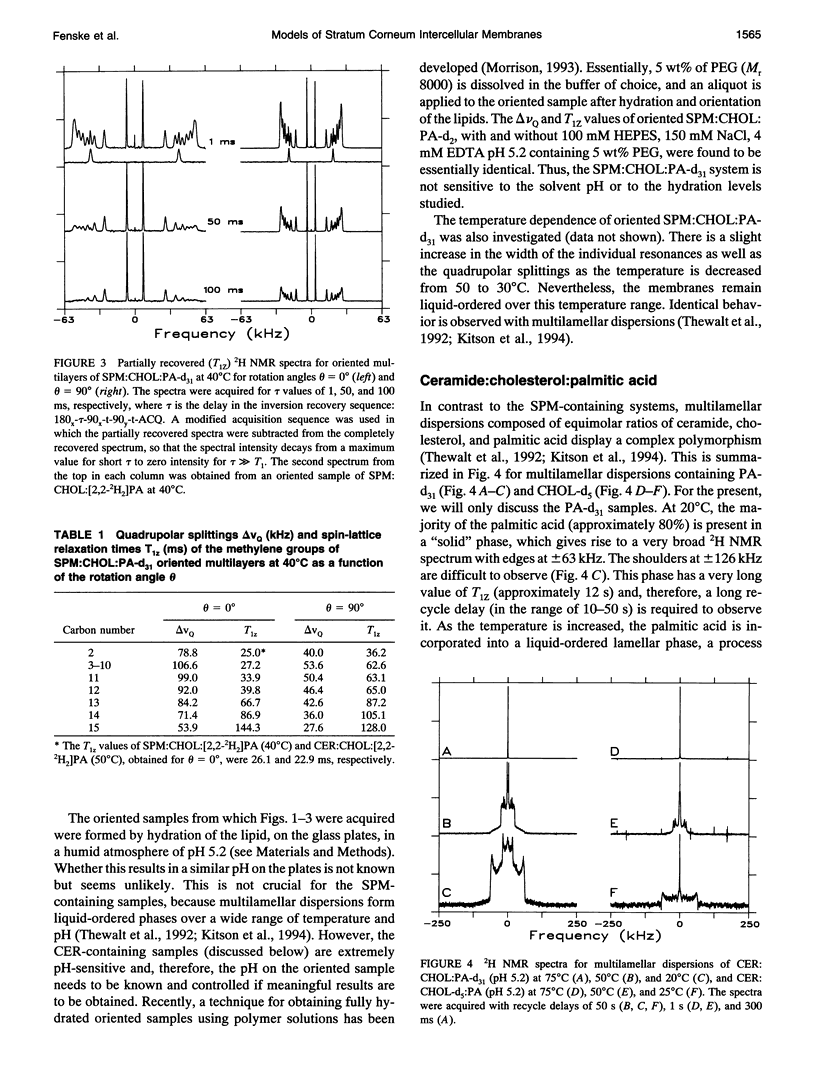
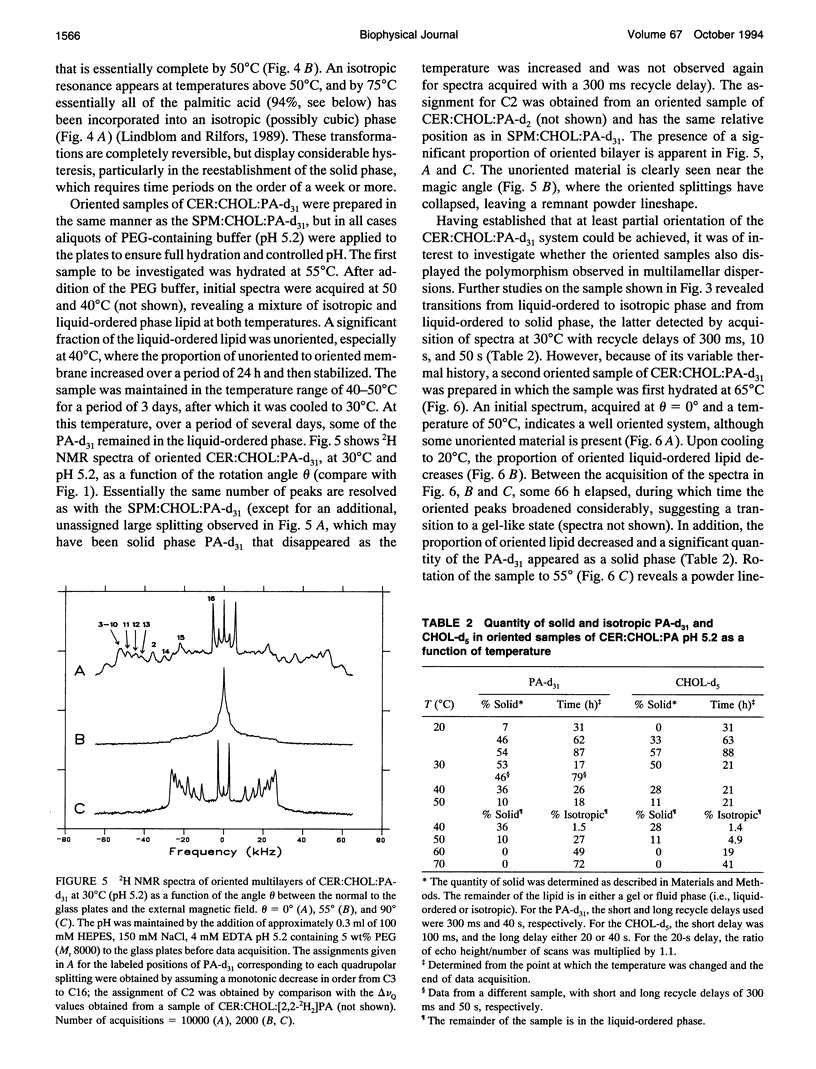
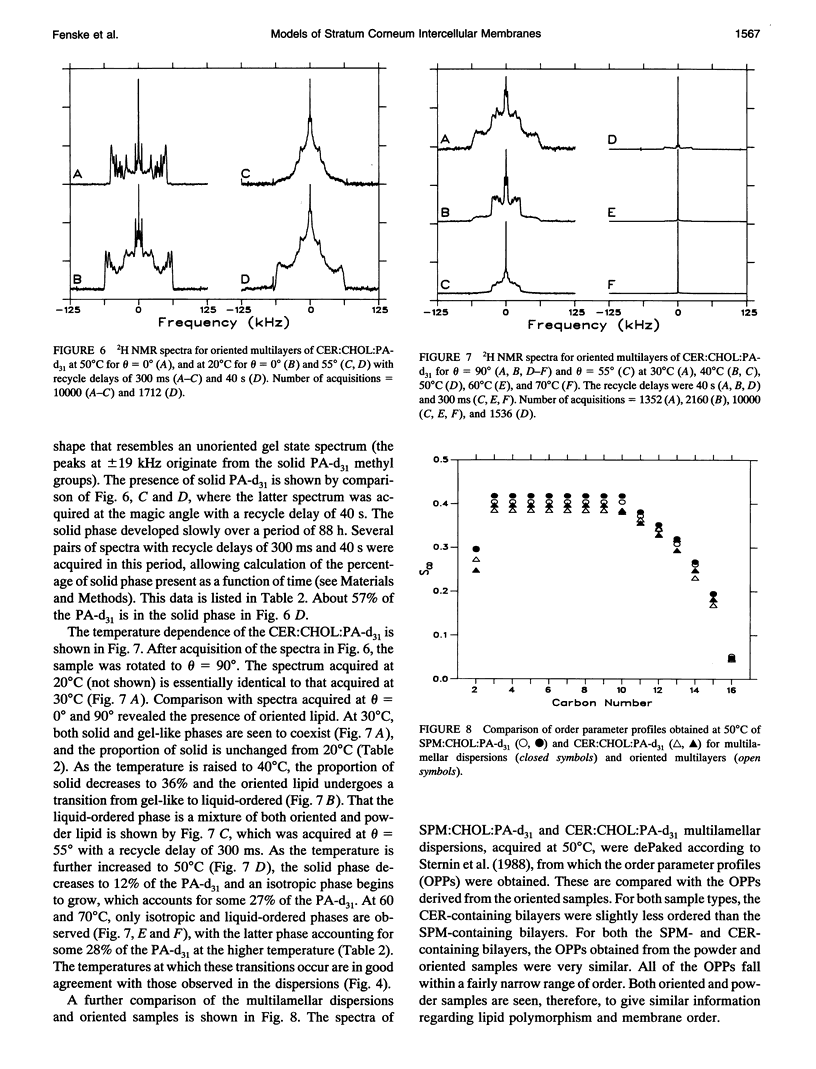
Deuterium NMR was used to characterize model membrane systems approximating the composition of the intercellular lipid lamellae of mammalian stratum corneum (SC). The SC models, equimolar mixtures of ceramide:cholesterol:palmitic acid (CER:CHOL:PA) at pH 5.2, were contrasted with the sphingomyelin:CHOL:PA (SPM:CHOL:PA) system, where the SPM differs from the CER only in the presence of a phosphocholine headgroup. The lipids were prepared both as oriented samples and as multilamellar dispersions, and contained either perdeuterated palmitic acid (PA-d31) or [2,2,3,4,6-2H5]CHOL (CHOL-d5). SPM:CHOL:PA-d31 formed liquid-ordered membranes over a wide range of temperatures, with a maximum order parameter of approximately 0.4 at 50 degrees C for positions C3-C10 (the plateau region). The quadrupolar splitting at C2 was significantly smaller, suggesting an orientational change at this position, possibly because of hydrogen bonding with water and/or other surface components. A comparison of the longitudinal relaxation times obtained at theta = 0 degrees and 90 degrees (where theta is the angle between the normal to the glass plates and the magnetic field) revealed a significant T1Z anisotropy for all positions. In contrast to the behavior observed with the SPM system, lipid mixtures containing CER exhibited a complex polymorphism. Between 20 and 50 degrees C, a significant portion of the entire membrane (as monitored by both PA-d31 and CHOL-d5) was found to exist as a solid phase, with the remainder either a gel or liquid-ordered phase. The proportion of solid decreased as the temperature was increased and disappeared entirely above 50 degrees C. Between 50 and 70 degrees C, the membrane underwent a liquid-ordered to isotropic phase transition. These transitions were reversible but displayed considerable hysteresis, especially the conversion from a fluid phase to solid. The order profiles, relaxation behavior, and angular dependence of these parameters suggest strongly that both the liquid-ordered CER- and SPM-membranes are bilayers. The unusual phase behavior observed for the CER-system, particularly the observation of solid-phase lipid at physiological temperatures, may provide insight into the functioning of the permeability barrier of stratum corneum.
Full text
PDF

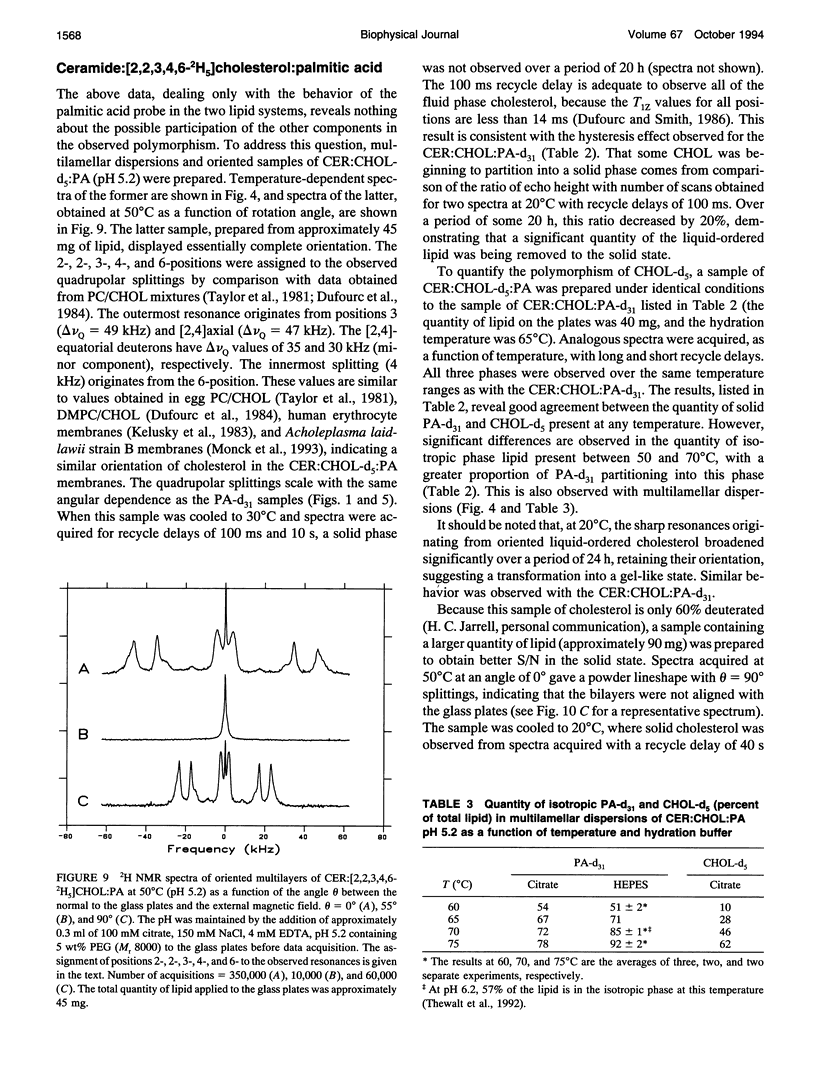
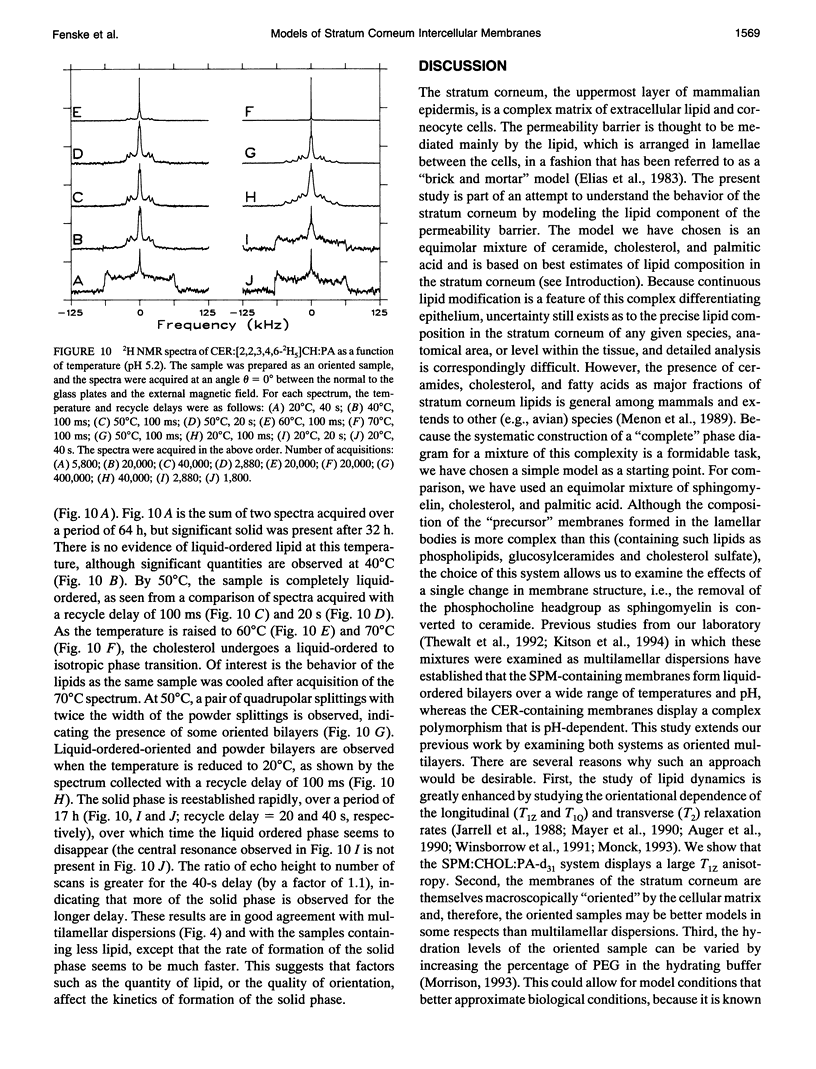




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W., Downing D. T. Deuterium NMR investigation of polymorphism in stratum corneum lipids. Biochim Biophys Acta. 1991 Sep 30;1068(2):189–194. doi: 10.1016/0005-2736(91)90209-q. [DOI] [PubMed] [Google Scholar]
- Bloom M., Evans E., Mouritsen O. G. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991 Aug;24(3):293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- Bouwstra J. A., Gooris G. S., van der Spek J. A., Bras W. Structural investigations of human stratum corneum by small-angle X-ray scattering. J Invest Dermatol. 1991 Dec;97(6):1005–1012. doi: 10.1111/1523-1747.ep12492217. [DOI] [PubMed] [Google Scholar]
- Bowser P. A., Gray G. M. Sphingomyelinase in pig and human epidermis. J Invest Dermatol. 1978 Jun;70(6):331–335. doi: 10.1111/1523-1747.ep12543516. [DOI] [PubMed] [Google Scholar]
- Chang F., Wertz P. W., Squier C. A. Comparison of glycosidase activities in epidermis, palatal epithelium and buccal epithelium. Comp Biochem Physiol B. 1991;100(1):137–139. doi: 10.1016/0305-0491(91)90096-v. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Bramhall J. Permeability of lipid bilayers to water and ionic solutes. Chem Phys Lipids. 1986 Jun-Jul;40(2-4):167–188. doi: 10.1016/0009-3084(86)90069-1. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Smith I. C. A detailed analysis of the motions of cholesterol in biological membranes by 2H-NMR relaxation. Chem Phys Lipids. 1986 Sep;41(2):123–135. doi: 10.1016/0009-3084(86)90004-6. [DOI] [PubMed] [Google Scholar]
- Fettiplace R., Haydon D. A. Water permeability of lipid membranes. Physiol Rev. 1980 Apr;60(2):510–550. doi: 10.1152/physrev.1980.60.2.510. [DOI] [PubMed] [Google Scholar]
- Freinkel R. K., Traczyk T. N. Lipid composition and acid hydrolase content of lamellar granules of fetal rat epidermis. J Invest Dermatol. 1985 Oct;85(4):295–298. doi: 10.1111/1523-1747.ep12276831. [DOI] [PubMed] [Google Scholar]
- Garson J. C., Doucet J., Lévêque J. L., Tsoucaris G. Oriented structure in human stratum corneum revealed by X-ray diffraction. J Invest Dermatol. 1991 Jan;96(1):43–49. doi: 10.1111/1523-1747.ep12514716. [DOI] [PubMed] [Google Scholar]
- Golden G. M., Guzek D. B., Harris R. R., McKie J. E., Potts R. O. Lipid thermotropic transitions in human stratum corneum. J Invest Dermatol. 1986 Mar;86(3):255–259. doi: 10.1111/1523-1747.ep12285373. [DOI] [PubMed] [Google Scholar]
- Golden G. M., Guzek D. B., Kennedy A. H., McKie J. E., Potts R. O. Stratum corneum lipid phase transitions and water barrier properties. Biochemistry. 1987 Apr 21;26(8):2382–2388. doi: 10.1021/bi00382a045. [DOI] [PubMed] [Google Scholar]
- Grayson S., Johnson-Winegar A. G., Wintroub B. U., Isseroff R. R., Epstein E. H., Jr, Elias P. M. Lamellar body-enriched fractions from neonatal mice: preparative techniques and partial characterization. J Invest Dermatol. 1985 Oct;85(4):289–294. doi: 10.1111/1523-1747.ep12276826. [DOI] [PubMed] [Google Scholar]
- Hsiao C. Y., Ottaway C. A., Wetlaufer D. B. Preparation of fully deuterated fatty acids by simple method. Lipids. 1974 Nov;9(11):913–915. doi: 10.1007/BF02532618. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Karlström G., Mouritsen O. G., Wennerström H., Zuckermann M. J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta. 1987 Nov 27;905(1):162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Jarrell H. C., Jovall P. A., Giziewicz J. B., Turner L. A., Smith I. C. Determination of conformational properties of glycolipid head groups by 2H NMR of oriented multibilayers. Biochemistry. 1987 Apr 7;26(7):1805–1811. doi: 10.1021/bi00381a003. [DOI] [PubMed] [Google Scholar]
- Kelusky E. C., Dufourc E. J., Smith I. C. Direct observation of molecular ordering of cholesterol in human erythrocyte membranes. Biochim Biophys Acta. 1983 Nov 9;735(2):302–304. doi: 10.1016/0005-2736(83)90306-1. [DOI] [PubMed] [Google Scholar]
- Kitson N., Thewalt J., Lafleur M., Bloom M. A model membrane approach to the epidermal permeability barrier. Biochemistry. 1994 May 31;33(21):6707–6715. doi: 10.1021/bi00187a042. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Cullis P. R., Bloom M. Modulation of the orientational order profile of the lipid acyl chain in the L alpha phase. Eur Biophys J. 1990;19(2):55–62. doi: 10.1007/BF00185086. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Fine B., Sternin E., Cullis P. R., Bloom M. Smoothed orientational order profile of lipid bilayers by 2H-nuclear magnetic resonance. Biophys J. 1989 Nov;56(5):1037–1041. doi: 10.1016/S0006-3495(89)82749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieckfeldt R., Villalain J., Gomez-Fernandez J. C., Lee G. Diffusivity and structural polymorphism in some model stratum corneum lipid systems. Biochim Biophys Acta. 1993 Aug 15;1150(2):182–188. doi: 10.1016/0005-2736(93)90088-h. [DOI] [PubMed] [Google Scholar]
- Menon G. K., Baptista L. F., Brown B. E., Elias P. M. Avian epidermal differentiation. II. Adaptive response of permeability barrier to water deprivation and replenishment. Tissue Cell. 1989;21(1):83–92. doi: 10.1016/0040-8166(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Monck M. A., Bloom M., Lafleur M., Lewis R. N., McElhaney R. N., Cullis P. R. Evidence for two pools of cholesterol in the Acholeplasma laidlawii strain B membrane: a deuterium NMR and DSC study. Biochemistry. 1993 Mar 30;32(12):3081–3088. doi: 10.1021/bi00063a020. [DOI] [PubMed] [Google Scholar]
- Morrison C. Polyethylene glycol as a hydration agent in oriented membrane bilayer samples. Biophys J. 1993 Apr;64(4):1063–1068. doi: 10.1016/S0006-3495(93)81472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts R. O., Francoeur M. L. The influence of stratum corneum morphology on water permeability. J Invest Dermatol. 1991 Apr;96(4):495–499. doi: 10.1111/1523-1747.ep12470197. [DOI] [PubMed] [Google Scholar]
- Rehfeld S. J., Elias P. M. Mammalian stratum corneum contains physiologic lipid thermal transitions. J Invest Dermatol. 1982 Jul;79(1):1–3. doi: 10.1111/1523-1747.ep12510367. [DOI] [PubMed] [Google Scholar]
- Rehfeld S. J., Plachy W. Z., Williams M. L., Elias P. M. Calorimetric and electron spin resonance examination of lipid phase transitions in human stratum corneum: molecular basis for normal cohesion and abnormal desquamation in recessive X-linked ichthyosis. J Invest Dermatol. 1988 Nov;91(5):499–505. doi: 10.1111/1523-1747.ep12476654. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Sternin E., Fine B., Bloom M., Tilcock C. P., Wong K. F., Cullis P. R. Acyl chain orientational order in the hexagonal HII phase of phospholipid-water dispersions. Biophys J. 1988 Oct;54(4):689–694. doi: 10.1016/S0006-3495(88)83004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewalt J., Kitson N., Araujo C., MacKay A., Bloom M. Models of stratum corneum intercellular membranes: the sphingolipid headgroup is a determinant of phase behavior in mixed lipid dispersions. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1247–1252. doi: 10.1016/0006-291x(92)91365-w. [DOI] [PubMed] [Google Scholar]
- Wertz P. W., Kremer M., Squier C. A. Comparison of lipids from epidermal and palatal stratum corneum. J Invest Dermatol. 1992 Mar;98(3):375–378. doi: 10.1111/1523-1747.ep12499809. [DOI] [PubMed] [Google Scholar]
- Wertz P. W., Madison K. C., Downing D. T. Covalently bound lipids of human stratum corneum. J Invest Dermatol. 1989 Jan;92(1):109–111. doi: 10.1111/1523-1747.ep13071317. [DOI] [PubMed] [Google Scholar]
- Wertz P. W., Swartzendruber D. C., Abraham W., Madison K. C., Downing D. T. Essential fatty acids and epidermal integrity. Arch Dermatol. 1987 Oct;123(10):1381–1384. [PubMed] [Google Scholar]
- White S. H., Mirejovsky D., King G. I. Structure of lamellar lipid domains and corneocyte envelopes of murine stratum corneum. An X-ray diffraction study. Biochemistry. 1988 May 17;27(10):3725–3732. doi: 10.1021/bi00410a031. [DOI] [PubMed] [Google Scholar]
- Winsborrow B. G., Smith I. C., Jarrell H. C. Dynamics of glycolipids in the liquid-crystalline state. 2H NMR study. Biophys J. 1991 Mar;59(3):729–741. doi: 10.1016/S0006-3495(91)82286-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley H. J., Summerly R. Lipid composition and metabolism in normal and diseased epidermis. Pharmacol Ther. 1981;13(2):357–383. doi: 10.1016/0163-7258(81)90006-1. [DOI] [PubMed] [Google Scholar]


