Abstract
By introducing a GAC anticodon, 21 different Escherichia coli tRNAs were misacylated with either phenylalanine or valine and assayed for their affinity to Thermus thermophilus elongation factor Tu (EF-Tu)⋅GTP by using a ribonuclease protection assay. The presence of a common esterified amino acid permits the thermodynamic contribution of each tRNA body to the overall affinity to be evaluated. The E. coli elongator tRNAs exhibit a wide range of binding affinities that varied from −11.7 kcal/mol for Val-tRNAGlu to −8.1 kcal/mol for Val-tRNATyr, clearly establishing EF-Tu⋅GTP as a sequence-specific RNA-binding protein. Because the ionic strength dependence of koff varied among tRNAs, some of the affinity differences are the results of a different number of phosphate contacts formed between tRNA and protein. Because EF-Tu is known to contact only the phosphodiester backbone of tRNA, the observed specificity must be a consequence of an indirect readout mechanism.
Elongation factor Tu (EF-Tu) binds GTP and aminoacyl tRNA (aa-tRNA) to form a ternary complex that subsequently binds ribosome and participates in codon-directed binding of the aa-tRNA to the ribosomal A site. Although EF-Tu⋅GTP binds poorly to tRNAs lacking the esterified amino acid (1), the protein is generally considered to lack specificity because it binds all elongator aa-tRNAs with a similar affinity (2–4). However, recent experiments have shown that EF-Tu⋅GTP exhibits substantial specificity for both the esterified amino acid and the tRNA body of aa-tRNAs (5). This specificity was previously unappreciated because the contributions of the amino acid and the tRNA body to the overall affinity are arranged in a compensatory manner such that the cognate aa-tRNAs all bind with similar affinities. However, misacylated tRNAs were found to bind EF-Tu⋅GTP with a wide range of affinities that were either tighter or weaker than the cognate aa-tRNAs (5). The four different tRNA bodies that were tested displayed about a 100-fold range of KD values with Thermus thermophilus EF-Tu⋅GTP when each was esterified with the same amino acid. The same range of KD values was observed when the four tRNAs were esterified with a different common amino acid, clearly establishing that EF-Tu shows specificity toward the tRNA body. However, the four tRNAs used in these experiments (Escherichia coli tRNAAla, tRNAGln, tRNAVal, and yeast tRNAPhe) have very similar overall architectures, raising the possibility that EF-Tu⋅GTP could exhibit a much larger range of affinities with tRNAs of different architectures.
Experiments presented here take advantage of the important role of the anticodon as a identity element for valyl-tRNA synthetase (ValRS) and phenylalanyl-tRNA synthetase (PheRS) (6–10) to prepare 21 different E. coli tRNAs that were misacylated with either valine or phenylalanine. By comparing the affinities of the different tRNAs esterified with a common amino acid, the specificity of EF-Tu⋅GTP for the different tRNA bodies was established.
Materials and Methods
E. coli tRNA genes inserted between the T7 promoter and a BstNI restriction site (11) were mutated to contain the GAC anticodon. For the four tRNAs that had a 5′ terminal C or U residue (tRNAPro, tRNAGln, tRNAAsn, and tRNAfMet), the T7 promoter was placed 10 nucleotides upstream of the native tRNA gene so that a precursor tRNA with a small 5′ extension was made. Transcription templates were prepared by PCR followed by BstNI digestion or, for those tRNAs with an internal BstNI site (tRNAHis, tRNAGlu, and tRNAfMet), by PCR using a T7 promoter primer and a primer that permits transcription termination at the tRNA terminus. In vitro transcription by T7 RNA polymerase was performed as described (11, 12) in the presence of 5′ GMP or 5′ AMP to ensure the presence of a 5′ terminal monophosphate. For the four precursor tRNAs, the transcription reactions were ethanol-precipitated and subjected to 5′ end processing in a reaction containing 50 mM Tris⋅HCl (pH 8.0), 75 mM MgCl2, 1.5 M NaOAc, 0.05% Triton X-100, and 0.8 μM Bacillus subtilis RNase P RNA at 37°C for 1 h (13). All tRNAs were purified on denaturing 20% polyacrylamide gels.
Aminoacylation reactions were performed with 1–2 μM tRNA, 4 mM ATP, 30 mM KCl, 15 mM MgCl2, 5 mM DTT, 30 mM Na-Hepes (pH 7.5), and either 20 μM [3H]Val (28 Ci/mmol) and 0.2–1 μM E. coli ValRS or 20 μM [3H]Phe (55 Ci/mmol) and 0.2–1 μM yeast PheRS. In most cases, 0.025 units/μl yeast inorganic pyrophosphatase (Sigma) was added to improve the aminoacylation yields (14). After incubation for 40 min at 37°C, 1/10 volume of 3 M NaOAc (pH 5.3) was added, and the reaction mixture was subjected to phenol/chloroform extraction and ethanol precipitation. The precipitate was dissolved in 5 mM NaOAc (pH 5.3) and stored at −80°C. In the case of tRNAPhe, the product of the aminoacylation reaction was used directly in EF-Tu binding experiments without phenol extraction and ethanol precipitation, because this procedure denatures unmodified E. coli tRNAPhe (15).
EF-Tu from T. thermophilus was overexpressed in E. coli and purified as described (16). EF-Tu⋅GTP was prepared immediately before use by incubating 1 μM EF-Tu⋅GDP, 3 mM phosphoenolpyruvate, 30 μg/ml pyruvate kinase, 10 mM DTT, 20 μM GTP, 20 mM MgCl2, 50 mM K-Hepes (pH 7.0), and 0.05–3.5 M NH4Cl at 37°C for 3 h (17). Dissociation rates were determined in 100 μl reactions by incubating 1 μM EF-Tu⋅GTP and <0.1 μM [3H]aa-tRNA in the same buffer for 20 min on ice to form the ternary complex. After the addition of 10 μl of 0.2 mg/ml RNase A, 10-μl aliquots were removed at various times, quenched into 100 μl of 10% trichloroacetic acid (TCA) containing 0.1 mg/ml of unfractionated tRNA, and filtered through a nitrocellulose membrane. Samples were washed and counted as described (16). Dissociation rates were measured at least three times at each NH4Cl concentration, and the mean value of koff was determined. Errors of slopes and KD values shown in Tables 2 and 3 are within 20% and 40%, respectively.
Table 2.
Binding properties of Val-tRNAs by T. thermophilus EF-Tu⋅GTP
| tRNA | KD [nM] | ΔG° [kcal/mol] | Slope of [NH4Cl] plot |
|---|---|---|---|
| tRNAAla | 4.3 | −10.5 | 0.87 |
| tRNAArg | 54 | −9.1 | 0.85 |
| tRNACys | 21 | −9.6 | 1.2 |
| tRNAGln | 250* | −8.3 | 0.95 |
| tRNAGly | 2.8 | −10.7 | 1.4 |
| 0.19† | −12.2 | ||
| tRNAIle | 110 | −8.7 | 0.76 |
| tRNALeu | 23 | −9.5 | 0.57 |
| tRNALys | 53 | −9.1 | 1.3 |
| tRNAMet | 33 | −9.4 | 0.83 |
| tRNAfMet | 180* | −8.4 | 0.68 |
| tRNAPhe | 48 | −9.2 | 1.2 |
| tRNAPro | 34 | −9.3 | 0.63 |
| tRNAThr | 4.0 | −10.5 | 1.4 |
| 0.66† | −11.5 | ||
| tRNATyr | 310* | −8.1 | 1.4 |
| tRNAVal | 92 | −8.8 | 0.69 |
KD values determined in 0.5 M NH4Cl, 2°C or extrapolated from ionic strength dependence (
) or temperature dependence (
) of KD.
Table 3.
Binding properties of Phe-tRNAs by T. thermophilus EF-Tu⋅GTP
| tRNA | KD [nM] | ΔG° [kcal/mol] | Slope of [NH4Cl] plot |
|---|---|---|---|
| tRNAAsn | 31 | −9.4 | 1.4 |
| tRNAAsp | 0.59* | −11.5 | 1.2 |
| 0.40† | −11.8 | ||
| tRNAGlu | 0.17* | −12.2 | 2.7 |
| 0.23† | −12.1 | ||
| tRNASec | 400* | −8.0 | 0.70 |
| tRNASer | 23 | −9.6 | 1.3 |
| tRNATrp | 65 | −9.0 | 0.99 |
| tRNAIle | 31* | −9.4 | 0.82 |
| tRNALys | 18* | −9.7 | 1.5 |
| tRNAMet | 15* | −9.8 | 0.85 |
| tRNAPhe | 21* | −9.6 | 1.1 |
| tRNATyr | 130 | −8.6 | 1.2 |
KD values determined in 0.5 M NH4Cl, 2°C or extrapolated from ionic strength dependence (
) or temperature dependence (
) of KD.
Results
Design and Misacylation of E. coli tRNAs.
To evaluate the tRNA specificity of EF-Tu, 22 different tRNA sequences were chosen, including one from each of the 20 isoacceptor groups of elongator tRNAs as well as tRNAfMet and tRNASec (Sec, selenocysteine; Table 1). Each tRNA was mutated to contain the tRNAVal anticodon, G34A35C36, and the C38A mutation was introduced into tRNAAsp and tRNAGlu. These mutations introduce important identity determinants expected to improve misacylation by E. coli ValRS and yeast PheRS (6–10, 18). Because EF-Tu binds to the acceptor stem and the T arm of tRNA (19, 20), these anticodon modifications are not expected to affect binding. Indeed, anticodon modifications of tRNAPhe, tRNAAla, and tRNAVal were found not to affect EF-Tu affinity (5).
Table 1.
Misacylation of unmodified E. coli tRNAs with GAC anticodon mutations by PheRS and ValRS
| tRNA | ValRS, % | PheRS, % | Ref. |
|---|---|---|---|
| tRNA2Ala | 153 | 25 | 40 |
| tRNA2Arg | 89 | 45 | 41 |
| tRNAAsn | 17 | 106 | 42 |
| tRNA1Asp | 19 | 82 | 42 |
| tRNACys | 96 | 28 | 43 |
| tRNA2Gln | 62 | 63 | 44 |
| tRNA2Glu | 10 | 26 | 45 |
| tRNA3Gly | 132 | 0 | 43 |
| tRNAHis | 0 | 0 | 12 |
| tRNA1Ile | 165 | 73 | 46 |
| tRNA1Leu | 74 | 6 | 47 |
| tRNALys | 103 | 82 | 41 |
| tRNAMet | 116 | 65 | 10 |
| tRNA1fMet | 133 | 73 | 48 |
| tRNAPhe | 111 | 78 | 49 |
| tRNA3Pro | 88 | 26 | 50 |
| tRNASec | 1 | 50 | 51 |
| tRNA1Ser | 2 | 69 | 52 |
| tRNA3Thr | 76 | 69 | 53 |
| tRNATrp | 50 | 102 | 54 |
| tRNA2Tyr | 115 | 58 | 52 |
| tRNA1Val | 108 | 58 | 10 |
All 22 tRNAs were prepared in unmodified form by using in vitro transcription with T7 RNA polymerase. Eighteen tRNAs were made in the conventional manner as an exact runoff transcript (11), and the four tRNAs with a 5′ terminal U or C were prepared by in vitro processing of a precursor tRNA with B. subtilis RNase P RNA. The tRNAs were tested for aminoacylation by both E. coli ValRS and yeast PheRS. Because the goal was only to obtain sufficient aa-tRNA for EF-Tu binding experiments, reactions contained high concentrations of aminoacyl-tRNA synthetase (aaRS) and pyrophosphatase to improve the aminoacylation yield. Although the activity of the tRNAs for the two aaRSs varied, 16 of the 22 tRNAs could be valylated to more than 50% and 19 of the 22 tRNAs could be phenylalanylated to more than 25% (Table 1). These levels are sufficient for EF-Tu binding experiments because the presence of deacylated tRNAs does not influence the binding affinity. Only tRNAHis was not aminoacylated by either aaRS, so binding experiments with this tRNA were not performed.
Binding of Val-tRNAs to T. thermophilus EF-Tu⋅GTP.
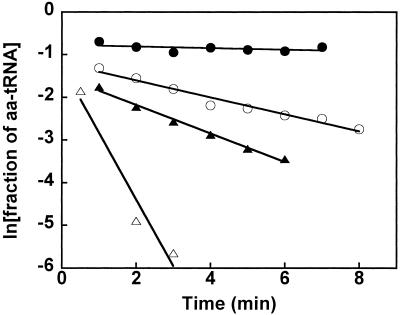
Fifteen tRNAs were aminoacylated with [3H]Val and mixed with 1 μM of T. thermophilus EF-Tu⋅GTP in 0.5 M NH4Cl buffer at 2°C. Under these conditions, the protein is in excess over the aa-tRNA and its concentration is high enough to achieve almost complete binding of the Val-tRNA. The stability of these complexes was determined by using an RNase protection assay (3) that makes use of the fact that the 3′ end of aa-tRNA is not susceptible to RNase digestion when bound to EF-Tu, thus the esterified [3H]Val remains acid-insoluble. A high enough concentration of RNase A was added to the reaction to cause the free [3H]Val-tRNA to be completely acid-soluble within 20 sec. Thus, the observed rate of disappearance of acid-insoluble radioactivity reflects the dissociation of the [3H]Val-tRNA from EF-Tu⋅GTP. As shown in Fig. 1, the dissociation rates vary dramatically among four of the Val-tRNAs in 0.5 M NH4Cl buffer. Some Val-tRNAs, such as Val-tRNAGln, dissociate so rapidly that it is difficult to obtain an accurate value by manual pipetting. Others, such as Val-tRNAGly, are extremely slow, also making it difficult to obtain an accurate koff value. In general, accurate koff values can most conveniently be obtained between 0.02 min−1 and 1 min−1.
Figure 1.
Time courses of RNase protection by T. thermophilus EF-Tu⋅GTP for Val-tRNAGly (●), Val-tRNAPro (○), Val-tRNAArg (▴), and Val-tRNAGln (▵) in 0.5M NH4Cl buffer at 2°C. Lines correspond to koff = 0.018, 0.20, 0.33, and 1.6 min−1, respectively.
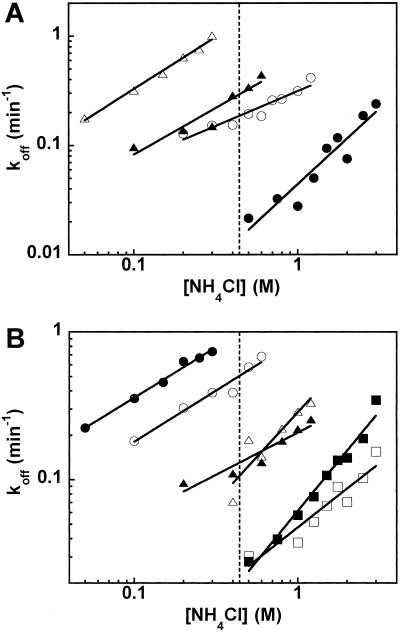
To obtain accurate koff values for Val-tRNAs that bind weakly or tightly, the NH4Cl concentration in the buffer was varied. As shown in Fig. 2, log koff increases linearly with increasing log [NH4Cl]. For the four Val-tRNAs in Fig. 1, accurate koff values could be obtained over an appropriate range of NH4Cl concentration (Fig. 2A). Thus, the koff for the tight binding of Val-tRNAGly could be determined at high salt concentrations, and the koff for the weak-binding Val-tRNAGln could be determined at low salt concentrations. By extrapolation of the linear plots, the koff values of all aa-tRNAs could be calculated at any desired ionic strength. To minimize extrapolation, a reference condition of 0.5 M NH4Cl was chosen to compare the aa-tRNAs. As shown in Fig. 2B for six other Val-tRNAs, the slopes of log koff vs. log [NH4Cl] plot vary significantly among the Val-tRNAs.
Figure 2.
(A) NH4Cl concentration dependence of dissociation rates at 2°C with Val-tRNAGly (●), Val-tRNAPro (○), Val-tRNAArg (▴), and Val-tRNAGln (▵). (B) Similar data with Val-tRNAfMet (●), Val-tRNAVal (○), Val-tRNALeu (▴), Val-tRNACys (▵), Val-tRNAThr (■), and Val-tRNAAla (□). Slopes of plots and KD values at 0.5 M NH4Cl (vertical dashed line) are given in Table 2.
Several Val-tRNAs bound EF-Tu so tightly that extremely high NH4Cl concentrations were required to determine the koff values. It therefore seemed prudent to use a second variable to estimate the EF-Tu binding affinity for these tRNAs. Because T. thermophilus EF-Tu is a thermostable protein, koff values of four tight-binding tRNAs, Val-tRNAGly, Val-tRNAThr, Phe-tRNAAsp, and Phe-tRNAGlu, were measured over a range of temperatures from 25°C to 45°C in the 0.5 M NH4Cl buffer. Previous experiments with several different aa-tRNAs over a wide range of NH4Cl concentrations and temperatures (5) have shown that koff values can be reliably converted to KD values by assuming a constant kon = 1.0 × 105 M−1 s−1 (17). After conversion to KD values, linear van't Hoff plots were observed (data not shown). These data permit extrapolation to 2°C to provide an alternative estimate of KD at the reference conditions of 2°C, 0.5 M NH4Cl. The resulting values are in good agreement with those obtained by extrapolating the NH4Cl concentration dependence of koff (Tables 2 and 3).
The binding properties of 15 Val-tRNAs with T. thermophilus EF-Tu⋅GTP are summarized in Table 2. The data of Val-tRNATrp could not be determined because, in addition to its relatively low level of valylation, its dissociation rate was too fast to be measured accurately even at the lowest salt concentration at which the protein remains active. It is clear that different tRNA bodies interact very differently with EF-Tu because the KD values vary by more than 100-fold. Tight-binding tRNAs include tRNAAla, tRNAGly, and tRNAThr, whereas tRNAGln and tRNATyr bind weakly. The slopes of the log koff vs. log [NH4Cl] plots also vary significantly.
Binding of the Phe-tRNAs to T. thermophilus EF-Tu⋅GTP.
The five tRNAs that could not be valylated, and the weak-binding tRNATrp were acylated with [3H]Phe, bound to EF-Tu⋅GTP, and their koff values measured as a function of NH4Cl concentration as described above. The data are summarized in Table 3. As with the Val-tRNAs, the different Phe-tRNAs exhibit different slopes and a range of KD values. However, it is not appropriate to compare the Val-tRNA and Phe-tRNA data sets directly because phenylalanine contributes more to the overall KD value than valine (5).
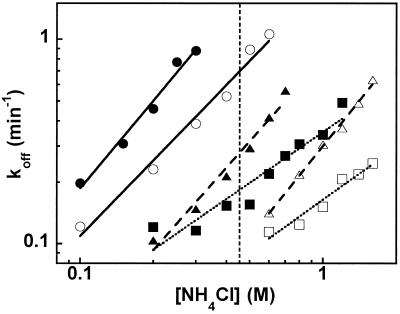
To determine the relative contribution of esterified phenylalanine and valine to the EF-Tu binding affinity, five tRNAs that could be acylated with both amino acids were chosen and their affinities as Phe-tRNAs determined (Table 3). Fig. 3 shows the log koff vs. log [NH4Cl] plots for tRNATyr, tRNALys, and tRNAMet acylated with valine or phenylalanine. Each tRNA binds EF-Tu more tightly when esterified with phenylalanine, but the slope is not affected by the esterified amino acid. These data confirm that phenylalanine contributes more than valine to protein binding and supports the view that the slope is defined by interaction with the tRNA body. When the difference of the ΔGo values of each Val-tRNA and Phe-tRNA was calculated, an average value of ΔΔG (Val-Phe) = 0.52 ± 0.14 kcal/mol was obtained for the five tRNAs tested. The SD is within the error of the measurement, supporting the conclusion that ΔΔG (Val-Phe) is independent of the tRNA body.
Figure 3.
NH4Cl concentration dependence of dissociation rates at 2°C with Val-tRNATyr (●), Phe-tRNATyr (○), and Val-tRNALys (▴), Phe-tRNALys (▵), Val-tRNAMet (■), and Phe-tRNAMet (□). Slopes of plots and KD values at 0.5 M NH4Cl (vertical dashed line) are given in Tables 2 and 3.
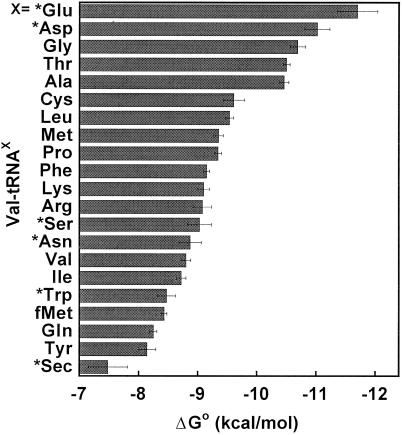
By using ΔΔG (Val-Phe), the experimental value of ΔGo for each Phe-tRNA in Table 3 can be converted to a calculated ΔGo for the same tRNA esterified with valine. This conversion permits comparison of the ΔGo values for all 21 tRNAs esterified with the same amino acid. The ΔGo values for E. coli Val-tRNAs are shown in Fig. 4 arranged in order of their affinities. The range of ΔGo values from the tightest (Val-tRNAGlu) to the weakest (Val-tRNASec) is about 4.2 kcal/mol. The tRNAs corresponding to negatively charged amino acids, tRNAGlu and tRNAAsp, are the tightest. The tRNAs whose cognate amino acids are small, such as tRNAGly and tRNAAla, also bind EF-Tu tightly. On the other hand, those tRNAs corresponding to large aromatic amino acids, such as tRNATrp and tRNATyr, bind weakly. The relative order of affinities for the different Val-tRNAs changes slightly at different NH4Cl concentrations because the slopes differ among the tRNAs. At the more physiological concentration of 150 mM NH4Cl, tRNAGln becomes the weakest elongator tRNA, whereas tRNAGlu remains the tightest.
Figure 4.
ΔGo of binding Val-tRNAs to T. thermophilus EF-Tu⋅GTP in 0.5 M NH4Cl buffer at 2°C presented in order of affinity. For amino acids marked by asterisks (*), ΔGo values of Phe-tRNAs are converted to those of Val-tRNAs by using ΔΔG (Val-Phe) = 0.52 kcal/mol.
Discussion
Experiments with 15 different E. coli tRNAs esterified with valine and 11 different E. coli tRNAs esterified with phenylalanine clearly establish that individual tRNA bodies contribute very differently to the overall binding affinity of aa-tRNAs to EF-Tu. Experiments with five different tRNAs esterified with both valine and phenylalanine showed that the contribution of the esterified amino acid to the overall binding affinity is independent of the tRNA body and permitted a hierarchy of binding free energies for 21 different valylated E. coli tRNAs to be established (Fig. 4). The observed range of free energies among the elongator tRNAs is 3.6 kcal/mol, which equals or exceeds the difference in the free energy of binding cognate and noncognate tRNAs by aaRSs (21, 22). Thus, EF-Tu should be regarded to be just as specific in binding tRNA as a typical aaRS.
The differential binding of the different tRNA bodies is consistent with the observation that the ionic strength dependence of the koff also varied substantially among different tRNA bodies. Linear plots of log koff vs. log [NH4Cl] were observed over a broad range of ionic strength, and the slopes varied from 0.57 to 2.7 among different tRNAs but were not altered by the identity of the amino acid. The slope of a log koff vs. log ionic strength plot can be interpreted as proportional to the number of phosphate contacts that are formed after protein binding (23, 24). Thus, tRNAs with a steep slope, such as Val-tRNAGlu, make more phosphate contacts with EF-Tu than tRNAs with a gentler slope, such as Val-tRNALeu. However, because there is only a weak correlation between the steepness of the slope and the total free energy of binding, tighter binding does not simply reflect the formation of additional phosphate contacts.
The broad range in binding affinities of T. thermophilus EF-Tu with the valylated E. coli tRNAs reported here contrasts with the much narrower range of binding affinities observed when the very similar E. coli EF-Tu protein binds the same set of tRNAs acylated with their cognate amino acid (3, 4). Taken together, the data suggest that the contributions of the esterified amino acids to the EF-Tu binding affinity must show a hierarchy that is roughly opposite to the tRNA hierarchy seen in Fig. 4. Thus, esterified glutamic acid and aspartic acid are expected to contribute relatively little to the EF-Tu binding affinity to compensate for the observed tight binding of tRNAGlu and tRNAAsp. In contrast, esterified glutamine and tyrosine are expected to contribute a lot to the binding affinity to offset the weaker binding of their corresponding tRNAs.
The experimental hierarchy of tRNA bodies and the deduced opposing hierarchy of amino acids help to explain why certain aa-tRNAs bind poorly to EF-Tu⋅GTP. For example, both the initiator tRNAfMet esterified with either methionine or formylmethionine and the specialized elongator tRNASec esterified with either serine or selenocysteine (Sec) show very weak binding to EF-Tu⋅GTP in vitro (1, 3, 4, 25). Because both these tRNAs enter translation by binding other proteins, this result is not unexpected. Experiments presented here show that Val-tRNASec and Val-tRNAfMet are indeed among the weakest aa-tRNAs tested. However, it is interesting that two valylated elongator tRNAs (tRNAGln and tRNATyr) bind EF-Tu with a similar low affinity. Thus, the poor binding of fMet(or Met)-tRNAfMet and Sec(or Ser)-tRNASec to EF-Tu is not simply because of poor binding of the tRNA bodies, but is also the result of a comparatively small contribution of their esterified amino acids. Gln-tRNAGln and Tyr-tRNATyr bind EF-Tu quite well because the esterified amino acids contribute strongly to the overall affinity.
A second example of EF-Tu discriminating against certain aa-tRNAs involves the misacylated Glu-tRNAGln and Asp-tRNAAsn that arise as intermediates in the synthesis of Gln-tRNAGln and Asn-tRNAAsn by a transamidation pathway present in many eubacteria and archaebacteria (26–29). Neither misacylated tRNA was found to bind the EF-Tu⋅GTP to an appreciable level even though the corresponding cognate aa-tRNAs bind well (30, 31). This observation can now be rationalized by the finding that the bodies of tRNAGln and tRNAAsn contribute comparatively less to EF-Tu affinity. Based on the tight binding of tRNAGlu and tRNAAsp the data also suggest that glutamic acid and aspartic acid are amino acids that bind comparatively weakly. As a result of having a weak tRNA and a weak amino acid, Glu-tRNAGln and Asp-tRNAAsn bind EF-Tu poorly until their esterified amino acids are converted to the tighter binding glutamine and asparagine by the transamidation pathway. In other words, the discrimination shown by EF-Tu against these misacylated tRNAs is a consequence of both the tRNA and amino acid-binding specificities intrinsic to the protein.
The molecular basis of the tRNA binding specificity displayed by T. thermophilus EF-Tu is not fully understood. The cocrystal structure of yeast Phe-tRNAPhe bound to the nearly identical Thermus aquaticus EF-Tu⋅GMPPNP reveals that the protein makes no direct contacts with the tRNA bases at all (20). A single contact with the conserved residue C75 is observed in the E. coli Cys-tRNACys cocrystal structure (19). The protein therefore contacts the tRNA almost entirely through the phosphodiester backbone of the conserved CCA terminus and helical residues of the acceptor and T stems. Eleven amino acids contact nine 2′ hydroxyl groups, and nine amino acids contact eight phosphate residues. This observation suggests that the high specificity of EF-Tu for tRNA is achieved through an “indirect readout” mechanism similar to that proposed for several DNA-binding proteins (32–34). Presumably, differences in binding energy are the result of small differences in the positions of phosphates and 2′ hydroxyls in the acceptor and T helices that arise as a consequence of the sequence. Additionally, sequence-dependent hydration patterns could also lead to affinity differences.
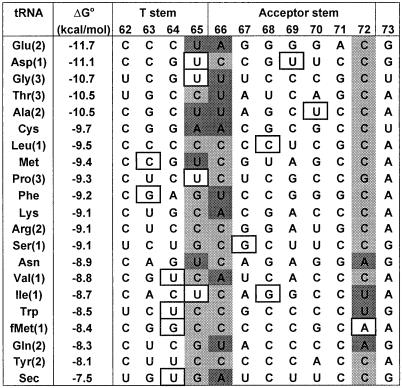
We have attempted to correlate the EF-Tu binding affinity of tRNAs with their sequence in the acceptor and T stems where the protein is known to bind. As shown in Fig. 5, no unique correlation with sequence is observed, suggesting that differences in the binding affinity arise from multiple contacts throughout the binding site. However, two interesting trends were observed. First, the tightest binding tRNAs tend to have AU and GU base pairs at positions 49–65 and 7–66 at the junction of the acceptor and T stems. Second, the weaker binding tRNAs tend to have a mismatch or AU pair at 1–72 and a GU pair at 50–64, which may prevent the formation of thermodynamically important 2′ hydroxyl contacts that form at positions 1 and 64 (16). Interestingly, all of these sites have previously been proposed to be “antideterminants” that reduce EF-Tu binding to tRNAfMet or tRNASec. Mutagenesis of tRNASec and tRNAAsp minihelices identified the sequence of the 50–64, 49–65, and 7–66 pairs as affecting EF-Tu binding affinity (35). Similarly, changing the weak C1–A72 in tRNAfMet to either a C1–G72 or U1–A72 pair improves EF-Tu binding (36). The correlations shown in Fig. 5 suggest that these positions should not simply be considered as antideterminants, but as determinants that are responsible for differences in the binding affinity of all tRNAs.
Figure 5.
Acceptor and T arm sequences of E. coli tRNAs that contact EF-Tu. All residues (except position 73) form normal base pairs except when the residue is boxed where GU or AC pairs are formed. Potentially important AU and GC pairs are highlighted in dark and light gray, respectively. The tRNA isoacceptor number (39) is given in parentheses.
An alternate way to understand the specificity exhibited by EF-Tu is that each tRNA makes a slightly different set of contacts with EF-Tu. This view is supported by the cocrystal structure of E. coli Cys-tRNACys with T. aquaticus EF-Tu⋅GMPPNP (19), which reveals several differences from the yeast Phe-tRNAPhe structure in the way that the phosphodiester backbone contacts the protein. For example, in the tRNAPhe structure, the 2′ hydroxyl of A66 contacts Lys-376 whereas in the tRNACys structure, this contact is not made and instead the phosphate of A66 contacts Gln-341, a contact not seen in the tRNAPhe structure. The possibility that different tRNAs make different protein contacts is supported by the data in Tables 2 and 3, which indicate substantial differences in the number of phosphate contacts formed between EF-Tu and the different E. coli tRNAs. However, several observations argue against such a model. First, because the tRNAPhe and tRNACys complexes were crystallized in different space groups, it is also possible that the observed differences in the protein-RNA contacts are the result of different packing constraints in the crystal lattice and do not reflect the interaction in solution (19, 20). Indeed, several of the 2′ hydroxyl contacts in the tRNAPhe structure, which differ from those in the tRNACys structure, do not contribute to the overall binding energy (16). Thus, not all of the observed differences in the two x-ray structures may be relevant to the specificity in solution. Second, Val-tRNAPhe binds EF-Tu with very similar affinity to Val-tRNACys, making it unclear whether different contacts would even be needed for these two tRNAs. Finally, another potentially important contribution to the differential binding of tRNAs is the free energy required to modify the structure or dynamics of the free tRNA so it adapts a uniform structure in the protein complex. Experiments with four different tRNAs modified with fluorescein at U8 suggest that although the environment around the fluorophore was quite different in the free tRNAs, it was quite similar after EF-Tu was bound (1). Interestingly, E. coli tRNAVal, which binds EF-Tu the weakest of the four tested, shows the largest fluorescence change whereas E. coli tRNAAla, which binds EF-Tu more tightly, shows a smaller fluorescence change. If the structure and dynamics of the tRNA in the free form are important for EF-Tu binding affinity, it is possible that parts of the tRNA remote from the acceptor–T stem-binding site could contribute to protein-binding affinity. Although most studies have suggested that this is not the case (17), at lease one remote tertiary interaction involving the 2′ hydroxyl of U7 seems to modulate the affinity of yeast tRNAPhe to EF-Tu (16). In summary, it is clear that the molecular basis of tRNA-binding specificity to EF-Tu is not yet understood and a thorough analysis of tightly and weakly binding tRNA bodies is required.
It seems that the sequences of tRNA are adjusted to thermodynamically compensate for the contribution of the different cognate amino acid side chains and thereby ensure uniform binding of all aa-tRNAs to EF-Tu. It has been suggested that the selective pressure for this thermodynamic compensation is to maximize translational accuracy because many misacylated tRNAs bind poorly to EF-Tu and thus will not be delivered to the ribosome (5). One problem with this view is that an equal number of misacylated tRNAs bind EF-Tu much tighter than the corresponding cognate aa-tRNA, and thus should decrease translation accuracy. The data presented here provide a possible explanation for this conundrum. As shown in Fig. 4, those tRNA bodies that bind EF-Tu tightly tend to have relatively small cognate amino acids and would only be expected to bind EF-Tu very tightly if misacylated with a larger amino acid. However, misacylated tRNAs of this type are less likely to form because large amino acid side chains are usually effectively sterically excluded by aaRSs (37). Thus, the specificity of EF-Tu for binding different tRNAs may have evolved to exclude a certain set of tRNAs that are prone to misacylation from binding ribosomes.
An alternate possibility is that thermodynamic compensation has evolved to ensure that all aa-tRNAs proceed through ribosomal decoding at a uniform rate. After the EF-Tu⋅GTP⋅aa-tRNA ternary complex binds the ribosome in a codon-directed manner, GTP hydrolysis occurs and aa-tRNA is released from EF-Tu⋅GDP and enters the ribosomal A site. This “accommodation” step may be rate limiting for peptide bond synthesis (38). Although the contacts between aa-tRNA and EF-Tu⋅GDP on the ribosome may be different from those in the ternary complex, it is likely that the differing thermodynamic contributions by the amino acid side chain and tRNA body would be maintained. If certain aa-tRNAs were to bind EF-Tu⋅GDP too tightly, accommodation and peptide bond formation would be too slow. In this view, the selective pressure to achieve uniform binding of the cognate aa-tRNAs is not to promote translational accuracy, but rather to ensure a uniform rate of translation. Thus, an analysis of the kinetic properties of misacylated tRNAs in translation would be valuable.
Acknowledgments
We thank J. Perona (Univ. of California, Santa Barbara) for E. coli ValRS. This work was supported by the National Institutes of Health Grant GM 37552 (to O.C.U.) and by a Postdoctoral Fellowship for Research Abroad by the Japan Society for the Promotion of Science (to H.A.).
Abbreviations
- aa-tRNA
aminoacyl-tRNA
- aaRS
aminoacyl-tRNA synthetase
- ValRS
valyl-tRNA synthetase
- Sec
selenocysteine
- EF-Tu
elongation factor Tu
- PheRS
phenylalanyl-tRNA synthetase
References
- 1.Janiak F, Dell V A, Abrahamson J K, Watson B S, Miller D L, Johnson A E. Biochemistry. 1990;29:4268–4277. doi: 10.1021/bi00470a002. [DOI] [PubMed] [Google Scholar]
- 2.Louie A, Ribeiro N S, Reid B R, Jurnak F. J Biol Chem. 1984;259:5010–5016. [PubMed] [Google Scholar]
- 3.Louie A, Jurnak F. Biochemistry. 1985;24:6433–6439. doi: 10.1021/bi00344a019. [DOI] [PubMed] [Google Scholar]
- 4.Ott G, Schiesswohl M, Kiesewetter S, Forster C, Arnold L, Erdmann V A, Sprinzl M. Biochim Biophys Acta. 1990;1050:222–225. doi: 10.1016/0167-4781(90)90170-7. [DOI] [PubMed] [Google Scholar]
- 5.LaRiviere F J, Wolfson A D, Uhlenbeck O C. Science. 2001;294:165–168. doi: 10.1126/science.1064242. [DOI] [PubMed] [Google Scholar]
- 6.Sampson J R, DiRenzo A B, Behlen L S, Uhlenbeck O C. Science. 1989;243:1363–1366. doi: 10.1126/science.2646717. [DOI] [PubMed] [Google Scholar]
- 7.Sampson J R, Behlen L S, DiRenzo A B, Uhlenbeck O C. Biochemistry. 1992;31:4161–4167. doi: 10.1021/bi00132a002. [DOI] [PubMed] [Google Scholar]
- 8.Tamura K, Himeno H, Asahara H, Hasegawa T, Shimizu M. Biochem Biophys Res Commun. 1991;177:619–623. doi: 10.1016/0006-291x(91)91833-x. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz J, Chu W-C, Derrick W B, Liu J C-H, Liu M, Yue D. Biochemistry. 1999;38:7737–7746. doi: 10.1021/bi990490b. [DOI] [PubMed] [Google Scholar]
- 10.Schulman L H, Pelka G. Science. 1988;242:765–768. doi: 10.1126/science.3055296. [DOI] [PubMed] [Google Scholar]
- 11.Sampson J R, Uhlenbeck O C. Proc Natl Acad Sci USA. 1988;85:1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himeno H, Hasegawa T, Ueda T, Watanabe K, Miura K, Shimizu M. Nucleic Acids Res. 1989;17:7855–7863. doi: 10.1093/nar/17.19.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J-L, Nolan J M, Harris M E, Pace N R. EMBO J. 1998;17:1515–1525. doi: 10.1093/emboj/17.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khvorova A, Motorin Y, Wolfson A D. Nucleic Acids Res. 1999;27:4451–4456. doi: 10.1093/nar/27.22.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington K M, Nazarenko I A, Dix D B, Thompson R C, Uhlenbeck O C. Biochemistry. 1993;32:7617–7622. doi: 10.1021/bi00081a003. [DOI] [PubMed] [Google Scholar]
- 16.Pleiss J A, Uhlenbeck O C. J Mol Biol. 2001;308:895–905. doi: 10.1006/jmbi.2001.4612. [DOI] [PubMed] [Google Scholar]
- 17.Nazarenko I A, Harrington K M, Uhlenbeck O C. EMBO J. 1994;13:2464–2471. doi: 10.1002/j.1460-2075.1994.tb06531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frugier M, Florentz C, Schimmel P, Giegé R. Biochemistry. 1993;32:14053–14061. doi: 10.1021/bi00213a039. [DOI] [PubMed] [Google Scholar]
- 19.Nissen P, Thirup S, Kjeldgaard M, Nyborg J. Structure Fold Des. 1999;7:143–156. doi: 10.1016/s0969-2126(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 20.Nissen P, Kjeldgaard M, Thirup S, Polekhina G, Reshetnikova L, Clark B F C, Nyborg J. Science. 1995;270:1464–1472. doi: 10.1126/science.270.5241.1464. [DOI] [PubMed] [Google Scholar]
- 21.Krauss G, Riesner D, Maass G. Eur J Biochem. 1976;68:81–93. doi: 10.1111/j.1432-1033.1976.tb10766.x. [DOI] [PubMed] [Google Scholar]
- 22.Lam S S M, Schimmel P R. Biochemistry. 1975;14:2775–2780. doi: 10.1021/bi00683a034. [DOI] [PubMed] [Google Scholar]
- 23.Lohman T M, de Haseth P L, Record M T., Jr Biochemistry. 1980;19:3522–3530. doi: 10.1021/bi00556a017. [DOI] [PubMed] [Google Scholar]
- 24.Record M T, Jr, Lohman T M, de Haseth P. J Mol Biol. 1976;107:145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- 25.Förster C, Ott G, Forchhammer K, Sprinzl M. Nucleic Acids Res. 1990;18:487–491. doi: 10.1093/nar/18.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tumbula D L, Becker H D, Chang W Z, Söll D. Nature (London) 2000;407:106–110. doi: 10.1038/35024120. [DOI] [PubMed] [Google Scholar]
- 27.Curnow A W, Ibba M, Söll D. Nature (London) 1996;382:589–590. doi: 10.1038/382589b0. [DOI] [PubMed] [Google Scholar]
- 28.Schön A, Kannangara C G, Gough S, Söll D. Nature (London) 1988;331:187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox M, Nirenberg M. Proc Natl Acad Sci USA. 1968;61:229–239. doi: 10.1073/pnas.61.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker H D, Kern D. Proc Natl Acad Sci USA. 1998;95:12832–12837. doi: 10.1073/pnas.95.22.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanzel M, Schön A, Sprinzl M. Eur J Biochem. 1994;219:435–439. doi: 10.1111/j.1432-1033.1994.tb19956.x. [DOI] [PubMed] [Google Scholar]
- 32.Bareket-Samish A, Cohen I, Haran T E. J Mol Biol. 1998;277:1071–1080. doi: 10.1006/jmbi.1998.1638. [DOI] [PubMed] [Google Scholar]
- 33.Leonard D A, Kerppola T K. Nat Struct Biol. 1998;5:877–881. doi: 10.1038/2316. [DOI] [PubMed] [Google Scholar]
- 34.Martin A M, Sam M D, Reich N O, Perona J J. Nat Struct Biol. 1999;6:269–277. doi: 10.1038/6707. [DOI] [PubMed] [Google Scholar]
- 35.Rudinger J, Hillenbrandt R, Sprinzl M, Giegé R. EMBO J. 1996;15:650–657. [PMC free article] [PubMed] [Google Scholar]
- 36.Seong B L, RajBhandary U L. Proc Natl Acad Sci USA. 1987;84:8859–8863. doi: 10.1073/pnas.84.24.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fersht A R, Dingwall C. Biochemistry. 1979;18:2627–2631. doi: 10.1021/bi00579a030. [DOI] [PubMed] [Google Scholar]
- 38.Pape T, Wintermeyer W, Rodnina M. EMBO J. 1999;18:3800–3807. doi: 10.1093/emboj/18.13.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komine Y, Adachi T, Inokuchi H, Ozeki H. J Mol Biol. 1990;212:579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- 40.Hou Y-M, Schimmel P. Nature (London) 1988;333:140–145. doi: 10.1038/333140a0. [DOI] [PubMed] [Google Scholar]
- 41.Tamura K, Himeno H, Asahara H, Hasegawa T, Shimizu M. Nucleic Acids Res. 1992;20:2335–2339. doi: 10.1093/nar/20.9.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nameki N, Tamura K, Himeno H, Asahara H, Hasegawa T, Shimizu M. Biochem Biophys Res Commun. 1992;189:856–862. doi: 10.1016/0006-291x(92)92282-3. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu M, Asahara H, Tamura K, Hasegawa T, Himeno H. J Mol Evol. 1992;35:436–443. doi: 10.1007/BF00171822. [DOI] [PubMed] [Google Scholar]
- 44.Jahn M, Rogers M J, Söll D. Nature (London) 1991;352:258–260. doi: 10.1038/352258a0. [DOI] [PubMed] [Google Scholar]
- 45.Sylvers L A, Rogers K C, Shimizu M, Ohtsuka E, Söll D. Biochemistry. 1993;32:3836–3841. doi: 10.1021/bi00066a002. [DOI] [PubMed] [Google Scholar]
- 46.Nureki O, Niimi T, Muramatsu T, Kanno H, Kohno T, Florentz C, Giegé R, Yokoyama S. J Mol Biol. 1994;236:710–724. doi: 10.1006/jmbi.1994.1184. [DOI] [PubMed] [Google Scholar]
- 47.Asahara H, Himeno H, Tamura K, Hasegawa T, Watanabe K, Shimizu M. J Mol Biol. 1993;231:219–229. doi: 10.1006/jmbi.1993.1277. [DOI] [PubMed] [Google Scholar]
- 48.Lee C-P, Dyson M, R, Mandal N, Varshney U, Bahramian B, RajBhandary U L. Proc Natl Acad Sci USA. 1992;89:9262–9266. doi: 10.1073/pnas.89.19.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson E T, Uhlenbeck O C. Biochemistry. 1992;31:10380–10389. doi: 10.1021/bi00157a028. [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Peterson R, Kessler J, Mussier-Forsyth K. Nucleic Acids Res. 1995;23:165–169. doi: 10.1093/nar/23.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudinger-Thirion J, Giegé R. RNA. 1999;5:495–502. doi: 10.1017/s1355838299981955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Himeno H, Hasegawa T, Ueda T, Watanabe K, Shimizu M. Nucleic Acids Res. 1990;18:6815–6819. doi: 10.1093/nar/18.23.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasegawa T, Miyano M, Himeno H, Sano Y, Kimura K, Shimizu M. Biochem Biophys Res Commun. 1992;184:478–484. doi: 10.1016/0006-291x(92)91219-g. [DOI] [PubMed] [Google Scholar]
- 54.Himeno H, Hasegawa T, Asahara H, Tamura K, Shimizu M. Nucleic Acids Res. 1991;19:6379–6382. doi: 10.1093/nar/19.23.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]