Abstract
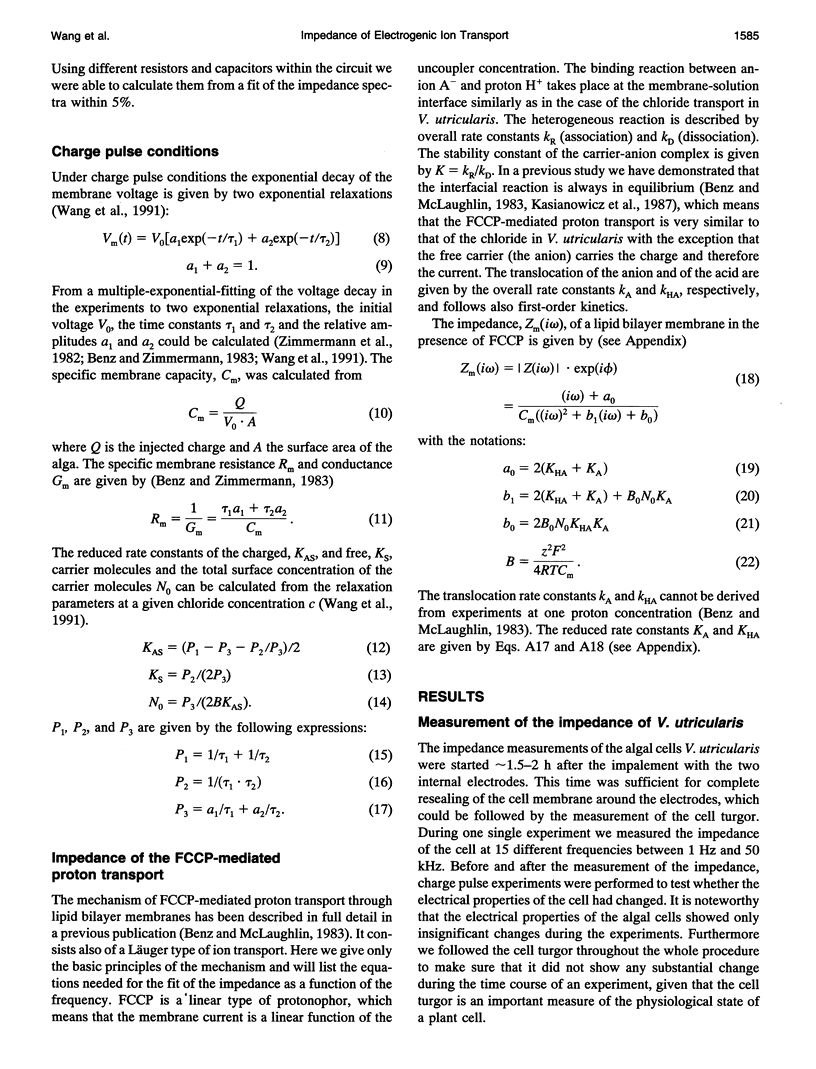
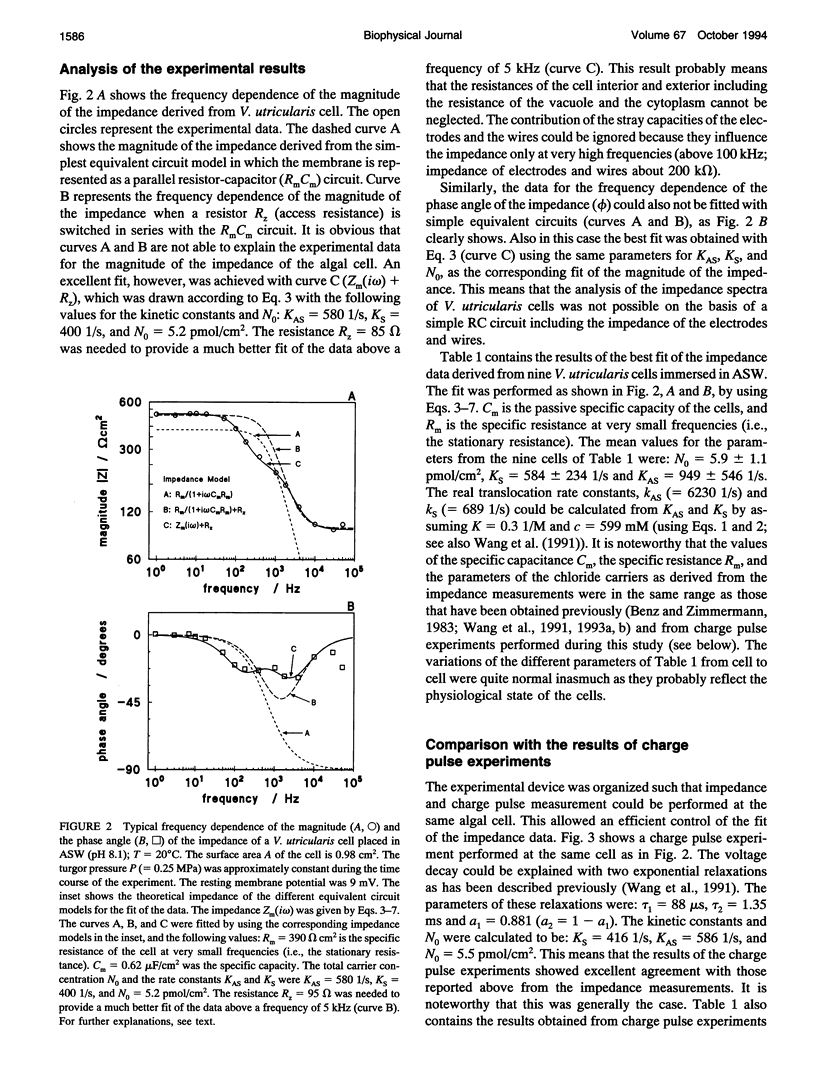
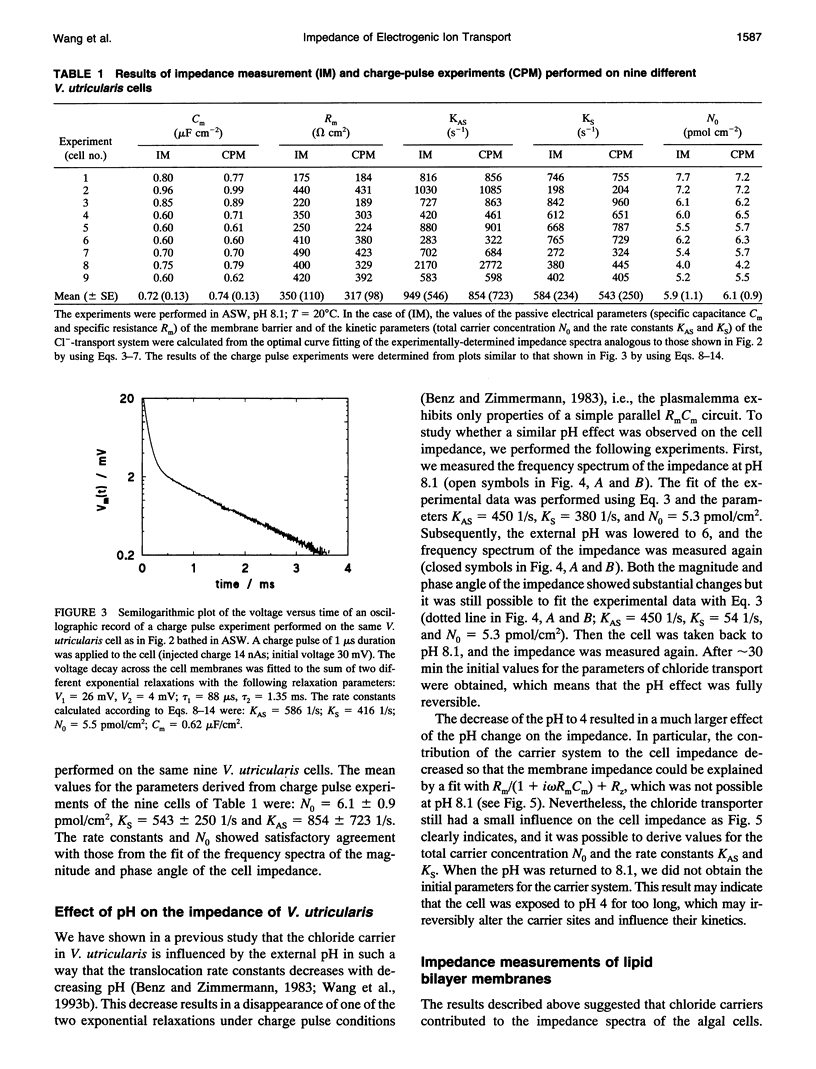
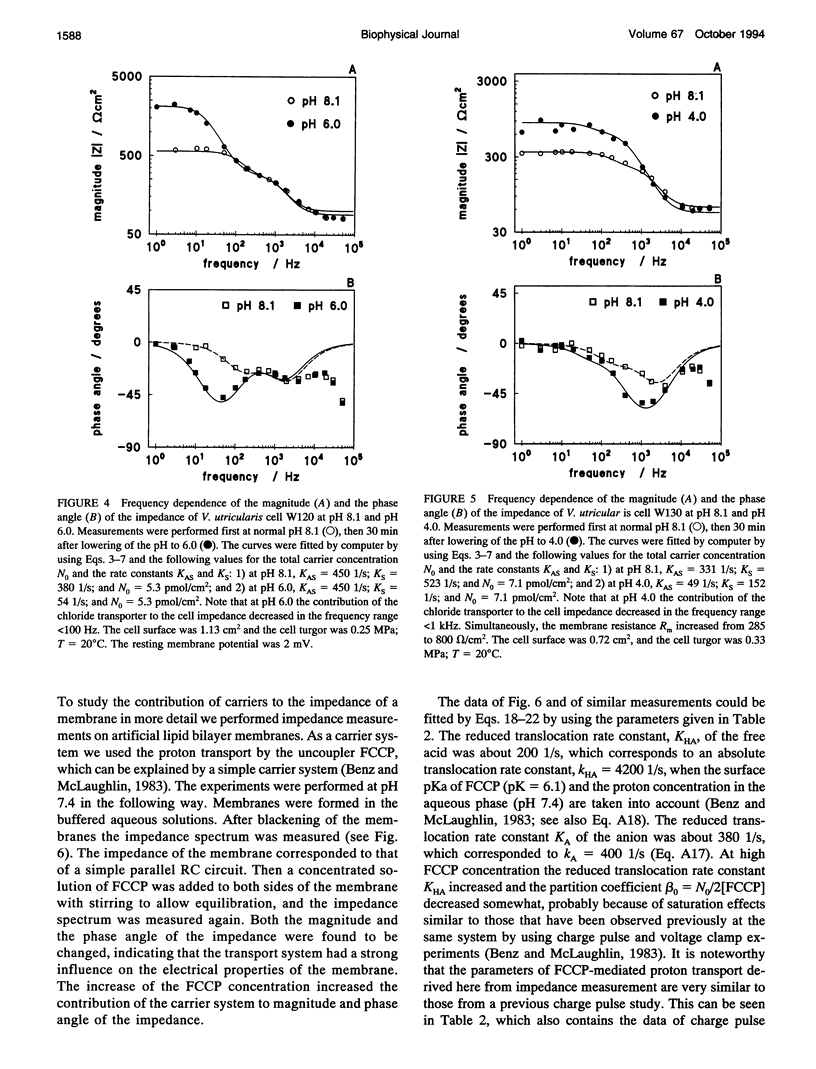
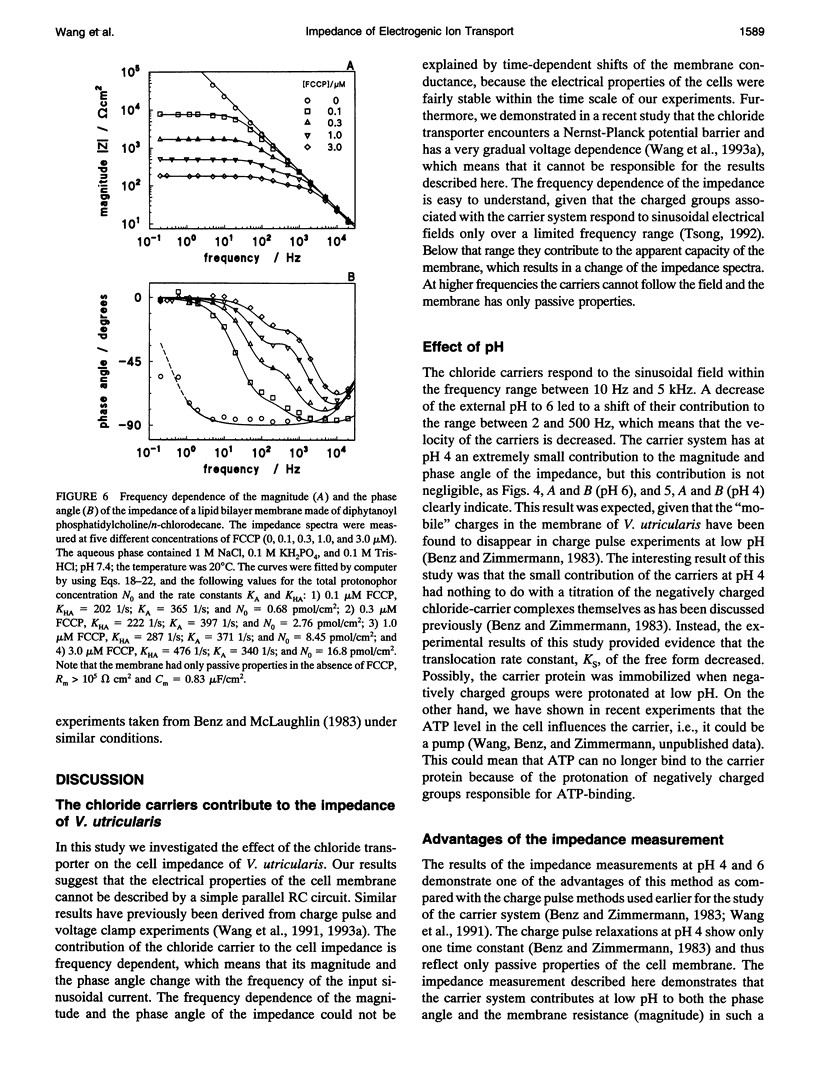
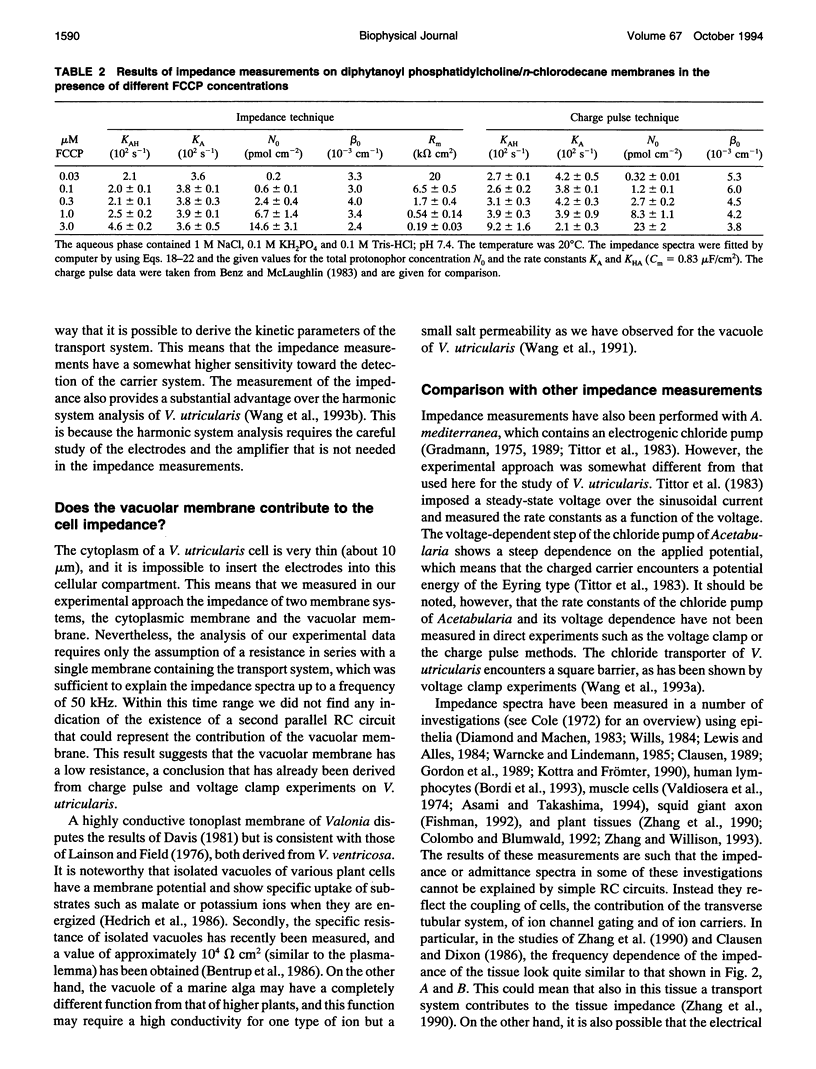
The cell membrane of Valonia utricularis contains an electrogenic carrier system for chloride (Wang et al., Biophys J. 59:235-248 (1991)). The electrical impedance of V. utricularis was measured in the frequency range between 1 Hz and 50 kHz. The analysis of the impedance spectra from V. utricularis and its comparison with equivalent circuit models showed that the transport system created a characteristic contribution to the impedance in the frequency range between 10 Hz and 5 kHz. The fit of the impedance spectra with the formalism derived from the theory of carrier-mediated transport allowed the determination of the kinetic parameters of chloride transport through the cell membrane of V. utricularis, and its passive electrical properties. Simultaneous measurements of the kinetic parameters with the charge pulse method demonstrated the equivalence of both experimental approaches with respect to the evaluation of the translocation rate constants of the free and the charged carriers and the total density of carriers within the membrane. Moreover, the impedance spectra of the protonophor-mediated proton transport by FCCP (carbonylcyanide p-trifluoromethoxyphenyl-hydrazone) were measured in model membranes. The carrier system made a substantial contribution to the impedance of the artificial membranes. The analysis of the spectra in terms of a simple carrier system (Benz and McLaughlin, 1983, Biophys. J. 41:381-398) allowed the evaluation of the kinetic and equilibrium parameters of the FCCP-mediated proton transport. The possible application of the measurement of impedance spectra for the study of biological transport systems is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asami K., Takashima S. Membrane admittance of cloned muscle cells in culture: use of a micropipet technique. Biochim Biophys Acta. 1994 Feb 23;1190(1):129–136. doi: 10.1016/0005-2736(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Bentrup F. W., Gogarten-Boekels M., Hoffmann B., Gogarten J. P., Baumann C. ATP-dependent acidification and tonoplast hyperpolarization in isolated vacuoles from green suspension cells of Chenopodium rubrum L. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2431–2433. doi: 10.1073/pnas.83.8.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Conti F. Structure of the squid axon membrane as derived from charge-pulse relaxation studies in the presence of absorbed lipophilic ions. J Membr Biol. 1981 Apr 15;59(2):91–104. doi: 10.1007/BF01875707. [DOI] [PubMed] [Google Scholar]
- Benz R., Läuger P. Kinetic analysis of carrier-mediated ion transport by the charge-pulse technique. J Membr Biol. 1976 Jun 9;27(1-2):171–191. doi: 10.1007/BF01869135. [DOI] [PubMed] [Google Scholar]
- Benz R., McLaughlin S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys J. 1983 Mar;41(3):381–398. doi: 10.1016/S0006-3495(83)84449-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Zimmermann U. Evidence for the presence of mobile charges in the cell membrane of Valonia utricularis. Biophys J. 1983 Jul;43(1):13–26. doi: 10.1016/S0006-3495(83)84318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F., Cametti C., Rosi A., Calcabrini A. Frequency domain electrical conductivity measurements of the passive electrical properties of human lymphocytes. Biochim Biophys Acta. 1993 Nov 21;1153(1):77–88. doi: 10.1016/0005-2736(93)90278-8. [DOI] [PubMed] [Google Scholar]
- Clausen C., Dixon T. E. Membrane electrical parameters in turtle bladder measured using impedance-analysis techniques. J Membr Biol. 1986;92(1):9–19. doi: 10.1007/BF01869011. [DOI] [PubMed] [Google Scholar]
- Clausen C. Impedance analysis in tight epithelia. Methods Enzymol. 1989;171:628–642. doi: 10.1016/s0076-6879(89)71035-1. [DOI] [PubMed] [Google Scholar]
- Davis R. F. Electrical Properties of the Plasmalemma and Tonoplast in Valonia ventricosa. Plant Physiol. 1981 Apr;67(4):825–831. doi: 10.1104/pp.67.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Gadsby D. C., Rakowski R. F. Voltage dependence of the Na-K pump. Annu Rev Physiol. 1988;50:225–241. doi: 10.1146/annurev.ph.50.030188.001301. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Machen T. E. Impedance analysis in epithelia and the problem of gastric acid secretion. J Membr Biol. 1983;72(1-2):17–41. doi: 10.1007/BF01870312. [DOI] [PubMed] [Google Scholar]
- Felle H. Amine transport at the plasmalemma of Riccia fluitans. Biochim Biophys Acta. 1980 Oct 16;602(1):181–195. doi: 10.1016/0005-2736(80)90300-4. [DOI] [PubMed] [Google Scholar]
- Fendler K., Grell E., Haubs M., Bamberg E. Pump currents generated by the purified Na+K+-ATPase from kidney on black lipid membranes. EMBO J. 1985 Dec 1;4(12):3079–3085. doi: 10.1002/j.1460-2075.1985.tb04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M. Assessment of conduction properties and thermal noise in cell membranes by admittance spectroscopy. Bioelectromagnetics. 1992;Suppl 1:87–100. doi: 10.1002/bem.2250130709. [DOI] [PubMed] [Google Scholar]
- Gordon L. G., Kottra G., Frömter E. Electrical impedance analysis of leaky epithelia: theory, techniques, and leak artifact problems. Methods Enzymol. 1989;171:642–663. doi: 10.1016/s0076-6879(89)71036-3. [DOI] [PubMed] [Google Scholar]
- Gradmann D. Analog circuit of the Acetabularia membrane. J Membr Biol. 1975 Dec 4;25(1-2):183–208. doi: 10.1007/BF01868574. [DOI] [PubMed] [Google Scholar]
- Hladky S. B. Ion transport and displacement currents with membrane-bound carriers: the theory for voltage-clamp currents, charge-pulse transients and admittance for symmetrical systems. J Membr Biol. 1979;46(3):213–237. doi: 10.1007/BF01868765. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H. Ion movements through the sodium pump. Annu Rev Physiol. 1985;47:535–544. doi: 10.1146/annurev.ph.47.030185.002535. [DOI] [PubMed] [Google Scholar]
- Kasianowicz J., Benz R., McLaughlin S. How do protons cross the membrane-solution interface? Kinetic studies on bilayer membranes exposed to the protonophore S-13 (5-chloro-3-tert-butyl-2'-chloro-4' nitrosalicylanilide). J Membr Biol. 1987;95(1):73–89. doi: 10.1007/BF01869632. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A. Gradient coupling in isolated intestinal cells. Fed Proc. 1981 Aug;40(10):2474–2479. [PubMed] [Google Scholar]
- Komor E., Tanner W. The determination of the membrane ptoential of Chlorella vulgaris. Evidence for electrogenic sugar transport. Eur J Biochem. 1976 Nov 1;70(1):197–204. doi: 10.1111/j.1432-1033.1976.tb10970.x. [DOI] [PubMed] [Google Scholar]
- Kottra G., Frömter E. Barium blocks cell membrane and tight junction conductances in Necturus gallbladder epithelium. Experiments with an extended impedance analysis technique. Pflugers Arch. 1990 Mar;415(6):718–725. doi: 10.1007/BF02584011. [DOI] [PubMed] [Google Scholar]
- Lainson R., Field C. D. Electrical properties of Valonia ventricosa. J Membr Biol. 1976 Oct 20;29(1-2):81–94. doi: 10.1007/BF01868953. [DOI] [PubMed] [Google Scholar]
- Liu D. S., Astumian R. D., Tsong T. Y. Activation of Na+ and K+ pumping modes of (Na,K)-ATPase by an oscillating electric field. J Biol Chem. 1990 May 5;265(13):7260–7267. [PubMed] [Google Scholar]
- Läuger P., Benz R., Stark G., Bamberg E., Jordan P. C., Fahr A., Brock W. Relaxation studies of ion transport systems in lipid bilayer membranes. Q Rev Biophys. 1981 Nov;14(4):513–598. doi: 10.1017/s003358350000247x. [DOI] [PubMed] [Google Scholar]
- Läuger P. Carrier-mediated ion transport. Science. 1972 Oct 6;178(4056):24–30. doi: 10.1126/science.178.4056.24. [DOI] [PubMed] [Google Scholar]
- Läuger P., Stark G. Kinetics of carrier-mediated ion transport across lipid bilayer membranes. Biochim Biophys Acta. 1970 Sep 15;211(3):458–466. doi: 10.1016/0005-2736(70)90251-8. [DOI] [PubMed] [Google Scholar]
- Markin V. S., Liu D., Gimsa J., Strobel R., Rosenberg M. D., Tsong T. Y. Ion channel enzyme in an oscillating electric field. J Membr Biol. 1992 Mar;126(2):137–145. doi: 10.1007/BF00231912. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986 Oct 16;323(6089):628–630. doi: 10.1038/323628a0. [DOI] [PubMed] [Google Scholar]
- Pickar A. D., Brown W. C. Capacitance of bilayers in the presence of lipophilic ions. Biochim Biophys Acta. 1983 Aug 24;733(1):181–185. doi: 10.1016/0005-2736(83)90104-9. [DOI] [PubMed] [Google Scholar]
- Rakowski R. F. Charge movement by the Na/K pump in Xenopus oocytes. J Gen Physiol. 1993 Jan;101(1):117–144. doi: 10.1085/jgp.101.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Astumian R. D. Kinetics of a multistate enzyme in a large oscillating field. Biophys J. 1990 Apr;57(4):689–696. doi: 10.1016/S0006-3495(90)82590-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Astumian R. D. Michaelis-Menten equation for an enzyme in an oscillating electric field. Biophys J. 1990 Oct;58(4):969–974. doi: 10.1016/S0006-3495(90)82441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Activation of electrogenic Rb+ transport of (Na,K)-ATPase by an electric field. J Biol Chem. 1984 Jun 10;259(11):7155–7162. [PubMed] [Google Scholar]
- Slayman C. L., Long W. S., Lu C. Y. The relationship between ATP and an electrogenic pump in the plasma membrane of Neurospora crassa. J Membr Biol. 1973;14(4):305–338. doi: 10.1007/BF01868083. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Slayman C. W. Depolarization of the plasma membrane of Neurospora during active transport of glucose: evidence for a proton-dependent cotransport system. Proc Natl Acad Sci U S A. 1974 May;71(5):1935–1939. doi: 10.1073/pnas.71.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y., Astumian R. D. Electroconformational coupling: how membrane-bound ATPase transduces energy from dynamic electric fields. Annu Rev Physiol. 1988;50:273–290. doi: 10.1146/annurev.ph.50.030188.001421. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Molecular recognition and processing of periodic signals in cells: study of activation of membrane ATPases by alternating electric fields. Biochim Biophys Acta. 1992 Mar 26;1113(1):53–70. doi: 10.1016/0304-4157(92)90034-8. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J. Sugar, amino acid, and Na+ cotransport in the proximal tubule. Annu Rev Physiol. 1979;41:181–195. doi: 10.1146/annurev.ph.41.030179.001145. [DOI] [PubMed] [Google Scholar]
- Valdiosera R., Clausen C., Eisenberg R. S. Impedance of frog skeletal muscle fibers in various solutions. J Gen Physiol. 1974 Apr;63(4):460–491. doi: 10.1085/jgp.63.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wehner G., Benz R., Zimmermann U. Harmonic system analysis of the algae Valonia utricularis: contribution of an electrogenic transport system to gain and phase-shift of the transfer function. Biophys J. 1993 Jun;64(6):1657–1667. doi: 10.1016/S0006-3495(93)81538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wehner G., Benz R., Zimmermann U. Influence of external chloride concentration on the kinetics of mobile charges in the cell membrane of Valonia utricularis: Evidence for the existence of a chloride carrier. Biophys J. 1991 Jan;59(1):235–248. doi: 10.1016/S0006-3495(91)82214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zimmermann U., Benz R. The voltage-dependent step of the chloride transporter of Valonia utricularis encounters a Nernst-Planck and not an Eyring type of potential energy barrier. Biophys J. 1993 Apr;64(4):1004–1016. doi: 10.1016/S0006-3495(93)81466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warncke J., Lindemann B. Voltage dependence of Na channel blockage by amiloride: relaxation effects in admittance spectra. J Membr Biol. 1985;86(3):255–265. doi: 10.1007/BF01870605. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Steudle E. The pressure-dependence of the hydraulic conductivity, the membrane resistance and membrane potential during turgor pressure regulation in Valonia utricularis. J Membr Biol. 1974;16(4):331–352. doi: 10.1007/BF01872422. [DOI] [PubMed] [Google Scholar]


