Abstract
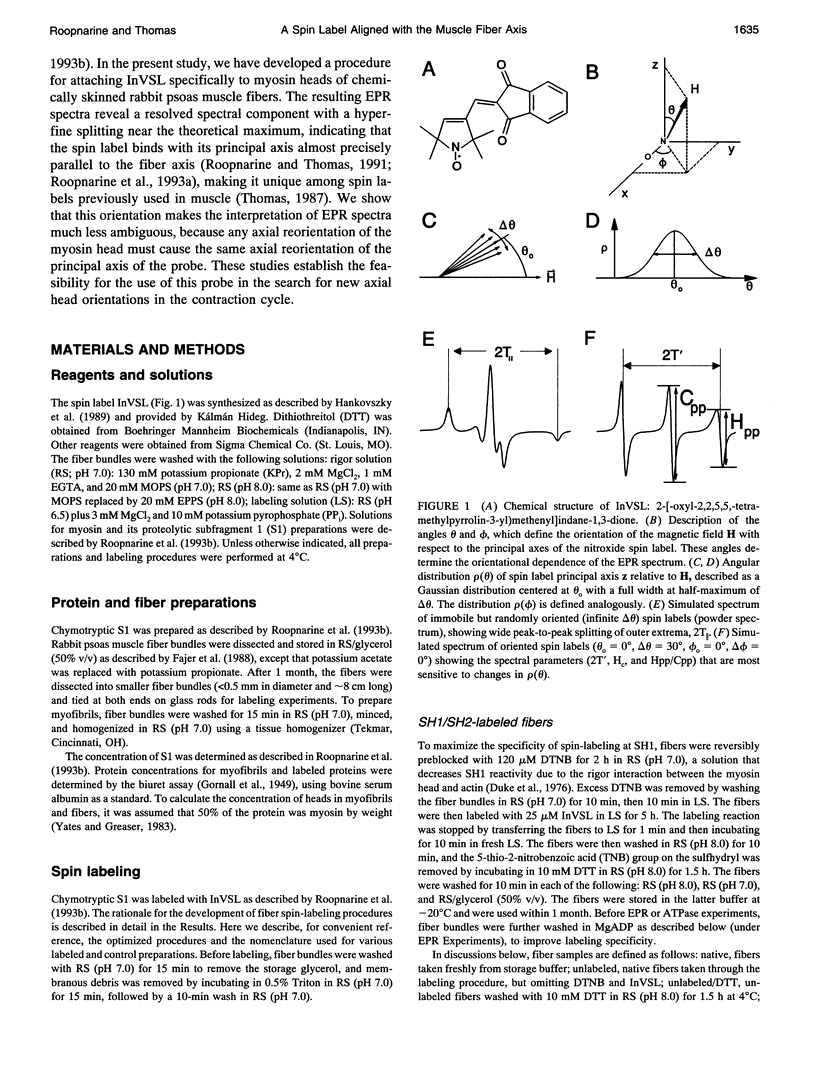
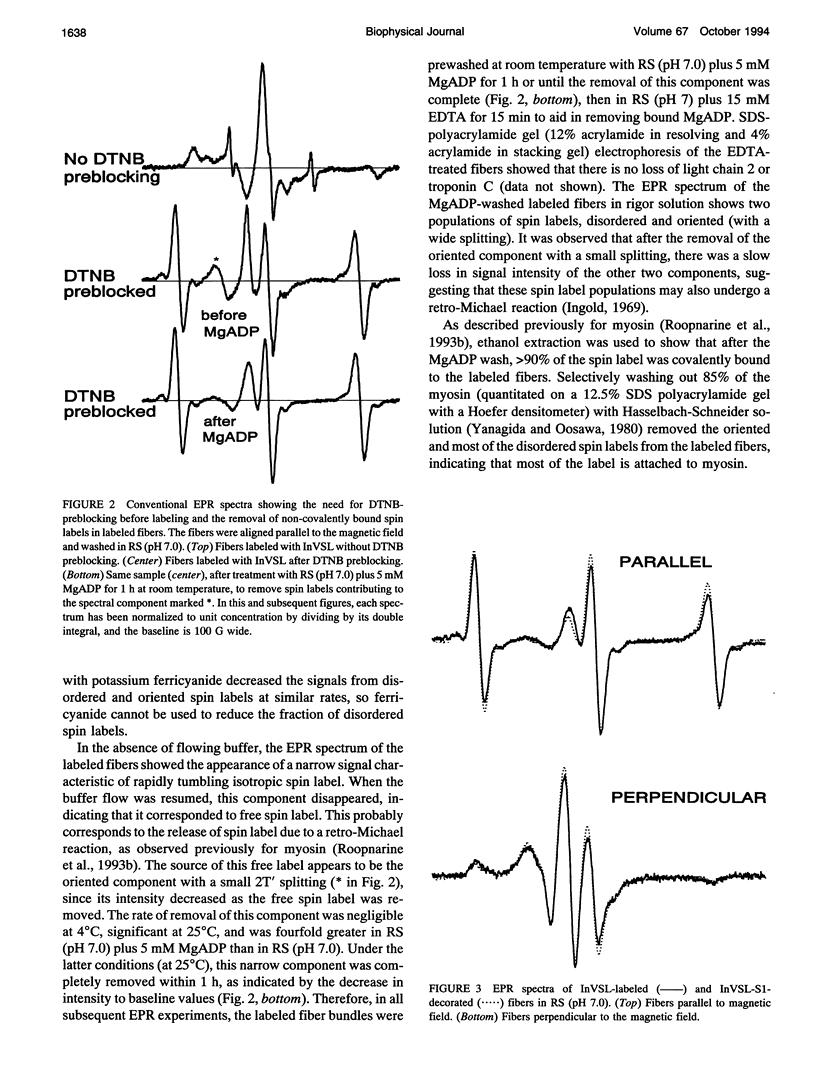
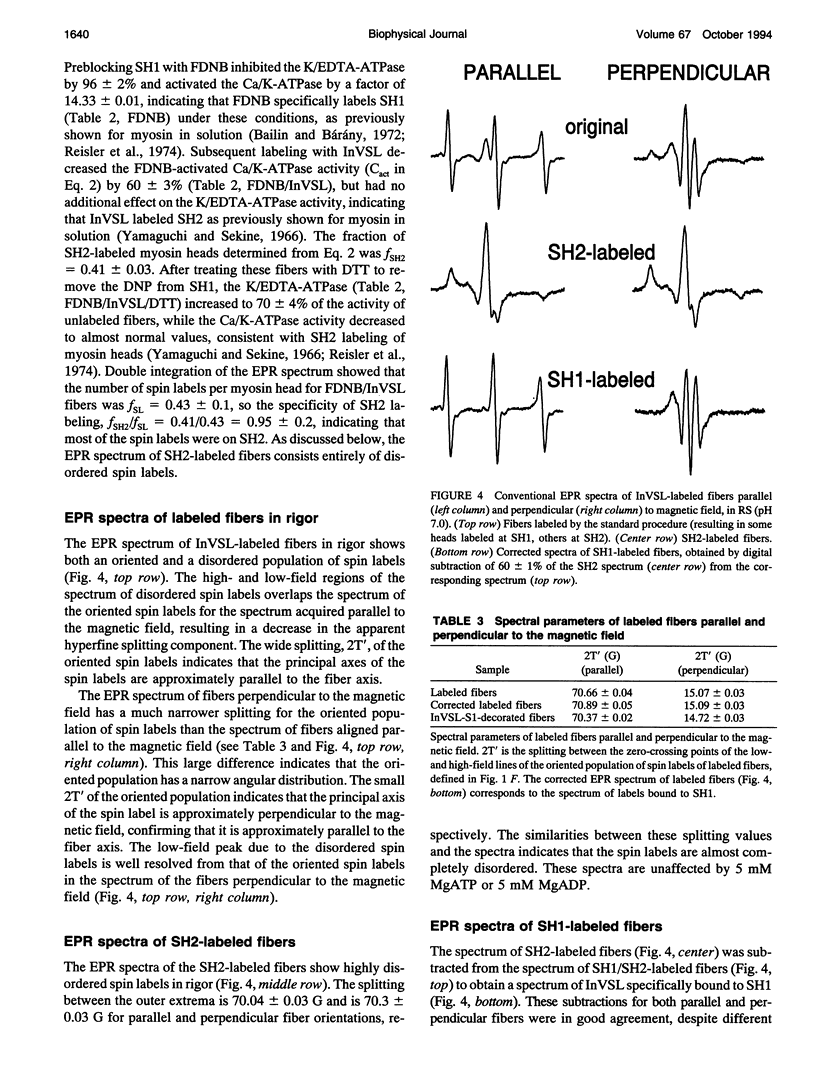
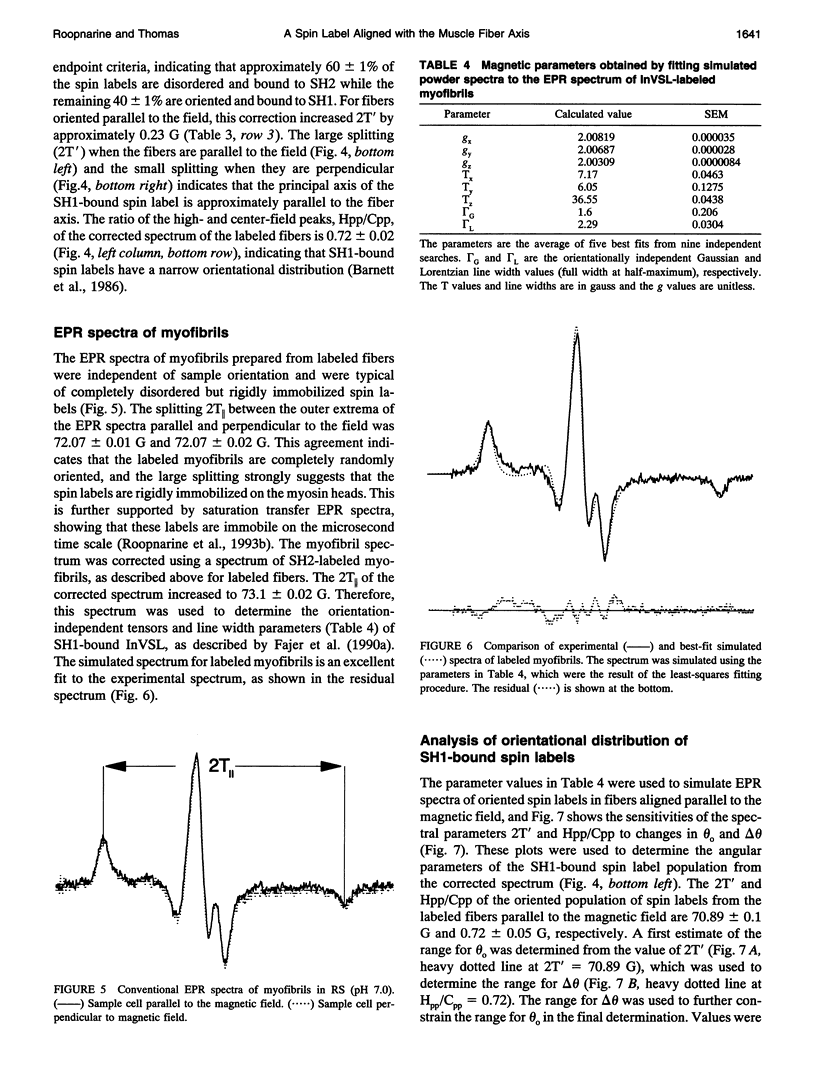
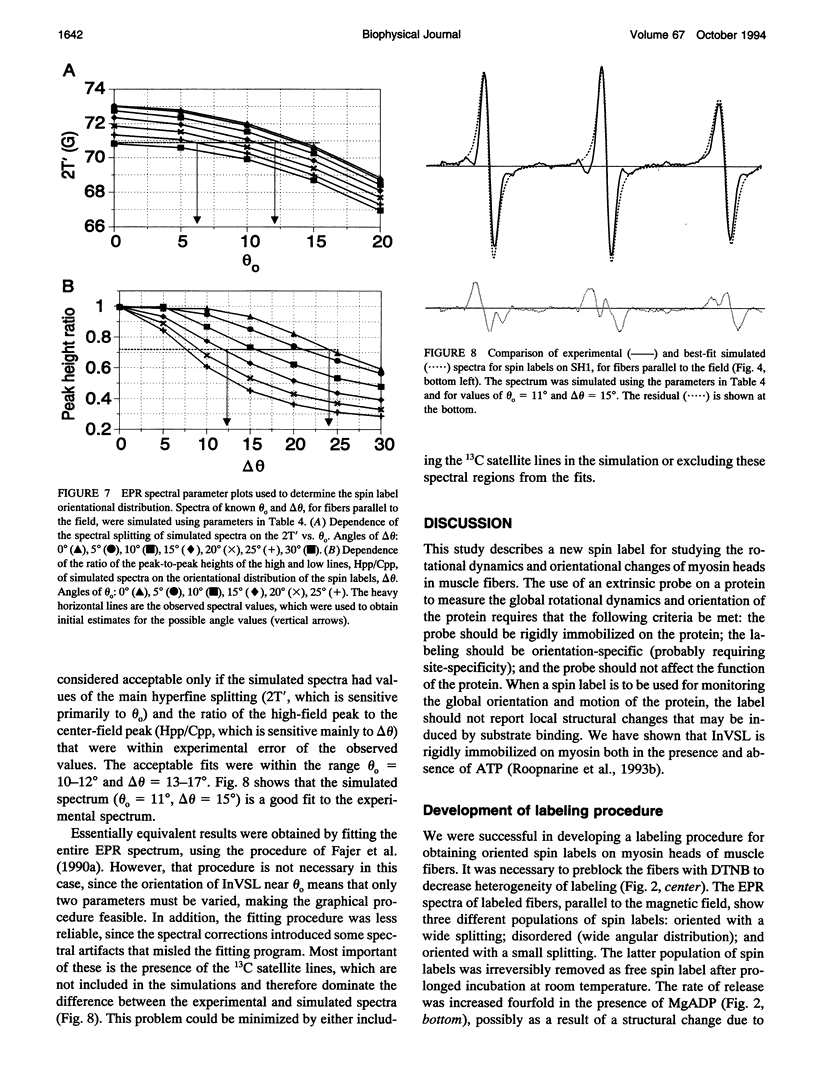
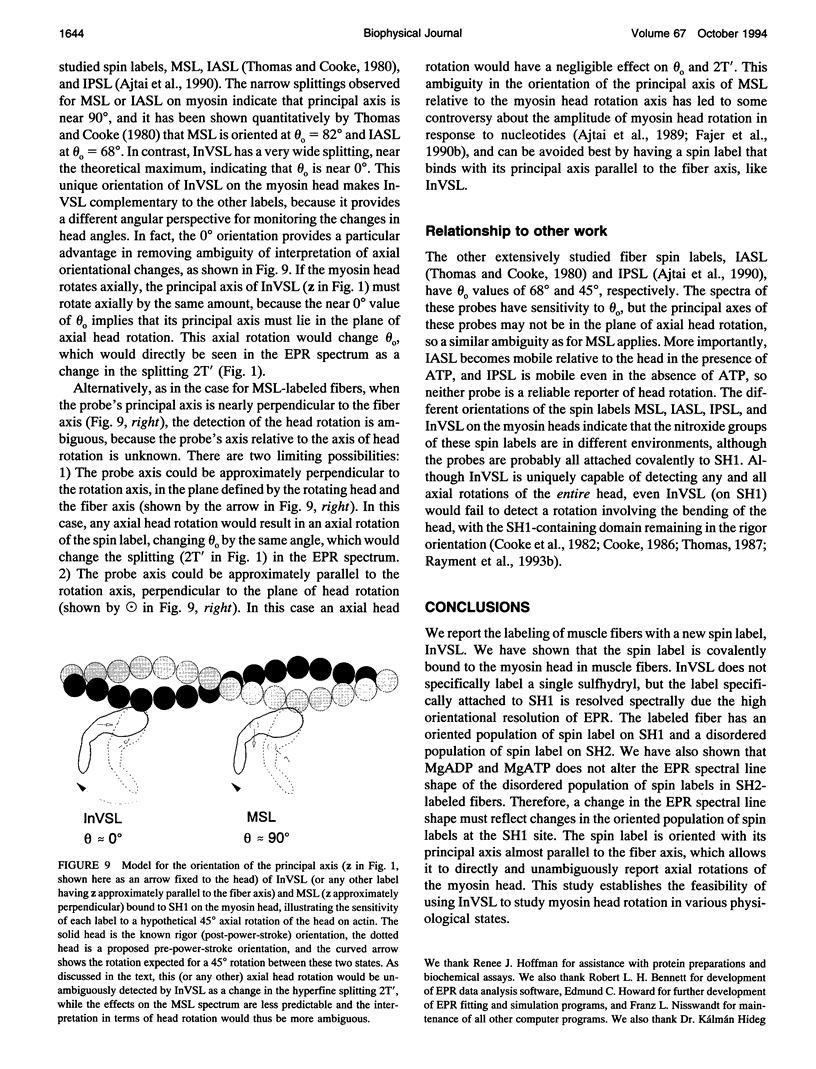
We have used an indane-dione spin label (2-[-oxyl-2,2,5,5-tetramethyl-3-pyrrolin-3-yl)methenyl]in dane-1,3-dione), designated InVSL, to study the orientation of myosin heads in bundles of chemically skinned rabbit psoas muscle fibers, with electron paramagnetic resonance (EPR) spectroscopy. After reversible preblocking with 5,5'-dithiobis(2-nitro-benzoic acid) (DTNB), we were able to attach most of the spin label covalently and rigidly to either Cys 707 (SH1) or Cys 697 (SH2) on myosin heads. EPR spectra of labeled fibers contained substantial contributions from both oriented and disordered populations of spin labels. Similar spectra were obtained from fibers decorated with InVSL-labeled myosin heads (subfragment 1), indicating that virtually all the spin labels in labeled fibers are on the myosin head. We specifically labeled SH2 with InVSL after reversible preblocking of the SH1 sites with 1-fluoro-2,4-dinitrobenzene (FDNB), resulting in a spectrum that indicated only disordered spin labels. Therefore, the oriented and disordered populations correspond to labels on SH1 and SH2, respectively. The spectrum of SH2-bound labels was subtracted to produce a spectrum corresponding to SH1-bound labels, which was used for further analysis. For this corrected spectrum, the angle between the fiber axis and the principal axis of the spin label was fitted well by a Gaussian distribution centered at theta o = 11 +/- 1 degree, with a full width at half-maximum of delta theta = 15 +/- 2 degrees. The unique orientation of InVSL, with its principal axis almost parallel to the fiber axis, makes it complementary to spin labels previously studied in this system. This label can provide unambiguous information about axial rotations of myosin heads, since any axial rotation of the head must be reflected in the same axial rotation of the principal axis of the probe, thus changing the hyperfine splitting. Therefore, InVSL-labeled fibers have ideal properties needed for further exploration myosin head orientation and rotational motion in muscle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajtai K., French A. R., Burghardt T. P. Myosin cross-bridge orientation in rigor and in the presence of nucleotide studied by electron spin resonance. Biophys J. 1989 Sep;56(3):535–541. doi: 10.1016/S0006-3495(89)82700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajtai K., Pótó L., Burghardt T. P. Specificity and orientation of (iodoacetamido)proxyl spin-labeled myosin subfragment 1 decorating muscle fibers: localization of protein-bound spin labels using SDS-PAGE. Biochemistry. 1990 Aug 21;29(33):7733–7741. doi: 10.1021/bi00485a023. [DOI] [PubMed] [Google Scholar]
- Bailin G., Bárány M. Thiolysis of dinitrophenylated myosin with restoration of adenosine triphosphatase activity. J Biol Chem. 1972 Dec 10;247(23):7815–7821. [PubMed] [Google Scholar]
- Barnett V. A., Fajer P., Polnaszek C. F., Thomas D. D. High-Resolution Detection of muscle Crossbridge Orientation by Electron Paramagnetic Resonance. Biophys J. 1986 Jan;49(1):144–147. doi: 10.1016/S0006-3495(86)83628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett V. A., Thomas D. D. Microsecond rotational motion of spin-labeled myosin heads during isometric muscle contraction. Saturation transfer electron paramagnetic resonance. Biophys J. 1989 Sep;56(3):517–523. doi: 10.1016/S0006-3495(89)82698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. L., Thomas D. D. Rotational dynamics of actin-bound intermediates of the myosin adenosine triphosphatase cycle in myofibrils. Biophys J. 1994 Jul;67(1):250–261. doi: 10.1016/S0006-3495(94)80476-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Crowder M. S., Cooke R. The effect of myosin sulphydryl modification on the mechanics of fibre contraction. J Muscle Res Cell Motil. 1984 Apr;5(2):131–146. doi: 10.1007/BF00712152. [DOI] [PubMed] [Google Scholar]
- Duke J., Takashi R., Ue K., Morales M. F. Reciprocal reactivities of specific thiols when actin binds to myosin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):302–306. doi: 10.1073/pnas.73.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmann M., Hankovszky H. O., Hideg K., Pedersen J. A., Marsh D. Vinyl ketone reagents for covalent protein modification. Nitroxide derivatives suited to rotational diffusion studies by saturation transfer electron spin resonance, using membrane-bound Na,K-ATPase as an example. Anal Biochem. 1990 Sep;189(2):274–282. doi: 10.1016/0003-2697(90)90120-x. [DOI] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Brunsvold N. J., Thomas D. D. Effects of AMPPNP on the orientation and rotational dynamics of spin-labeled muscle cross-bridges. Biophys J. 1988 Apr;53(4):513–524. doi: 10.1016/S0006-3495(88)83131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Matta J. J., Thomas D. D. Effect of ADP on the orientation of spin-labeled myosin heads in muscle fibers: a high-resolution study with deuterated spin labels. Biochemistry. 1990 Jun 19;29(24):5865–5871. doi: 10.1021/bi00476a031. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- KIELLEY W. W., BRADLEY L. B. The relationship between sulfhydryl groups and the activation of myosin adenosinetriphosphatase. J Biol Chem. 1956 Feb;218(2):653–659. [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reisler E., Burke M., Harrington W. F. Cooperative role of two sulfhydryl groups in myosin adenosine triphosphatase. Biochemistry. 1974 May 7;13(10):2014–2022. doi: 10.1021/bi00707a003. [DOI] [PubMed] [Google Scholar]
- Roopnarine O., Hideg K., Thomas D. D. Saturation transfer electron parametric resonance of an indane-dione spin-label. Calibration with hemoglobin and application to myosin rotational dynamics. Biophys J. 1993 Jun;64(6):1896–1907. doi: 10.1016/S0006-3495(93)81561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKINE T., BARNETT L. M., KIELLEY W. W. The active site of myosin adenosine triphosphatase. I. Localization of one of the sulfhydryl groups. J Biol Chem. 1962 Sep;237:2769–2772. [PubMed] [Google Scholar]
- Seidel J. C. Similar effects on enzymic activity due to chemical modification of either of two sulfhydryl groups of myosin. Biochim Biophys Acta. 1969 May;180(1):216–219. doi: 10.1016/0005-2728(69)90215-1. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D. Spectroscopic probes of muscle cross-bridge rotation. Annu Rev Physiol. 1987;49:691–709. doi: 10.1146/annurev.ph.49.030187.003355. [DOI] [PubMed] [Google Scholar]
- Wilson M. G., Mendelson R. A. A comparison of order and orientation of crossbridges in rigor and relaxed muscle fibres using fluorescence polarization. J Muscle Res Cell Motil. 1983 Dec;4(6):671–693. doi: 10.1007/BF00712160. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Sekine T. Sulfhydryl groups involved in the active site of myosin A adenosine triphosphatase. I. Specific blocking of the SH group responsible for the inhibitory phase in "B phasic response" of the catalytic activity. J Biochem. 1966 Jan;59(1):24–33. doi: 10.1093/oxfordjournals.jbchem.a128254. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Oosawa F. Conformational changes of F-actin-epsilon-ADP in thin filaments in myosin-free muscle fibers induced by Ca2+. J Mol Biol. 1980 Jun 25;140(2):313–320. doi: 10.1016/0022-2836(80)90108-4. [DOI] [PubMed] [Google Scholar]
- Yates L. D., Greaser M. L. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983 Jul 25;168(1):123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]



