Abstract
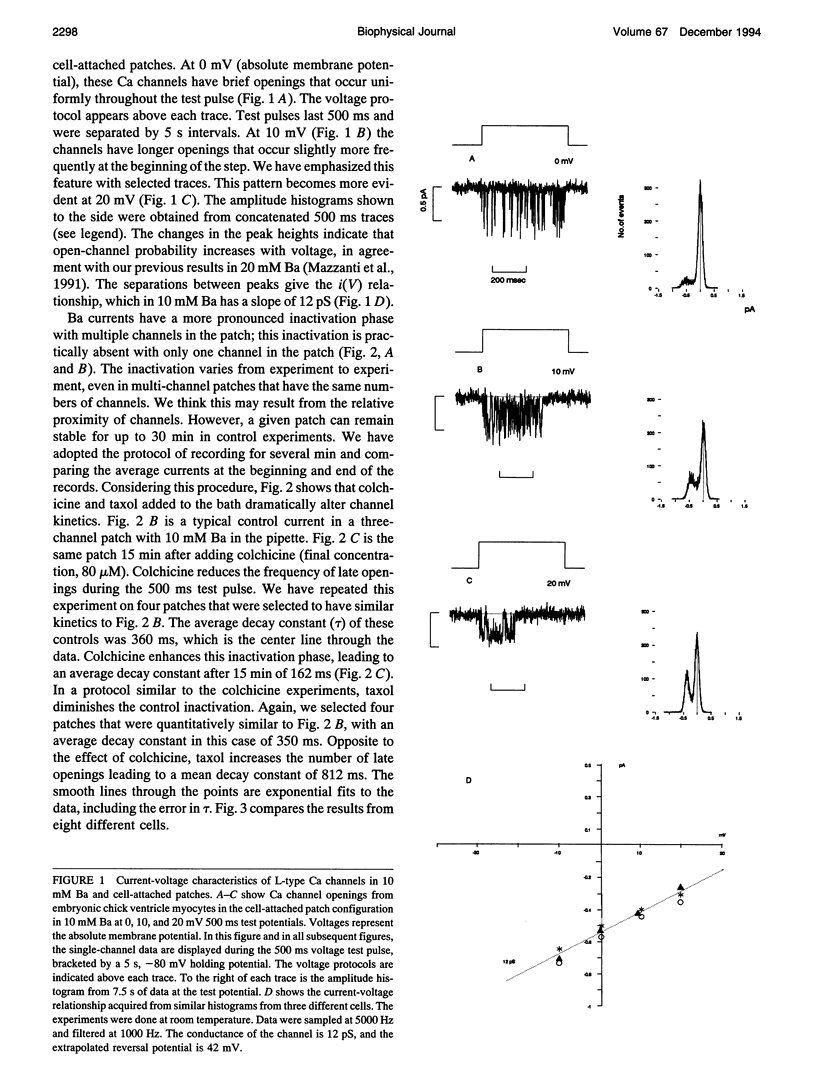
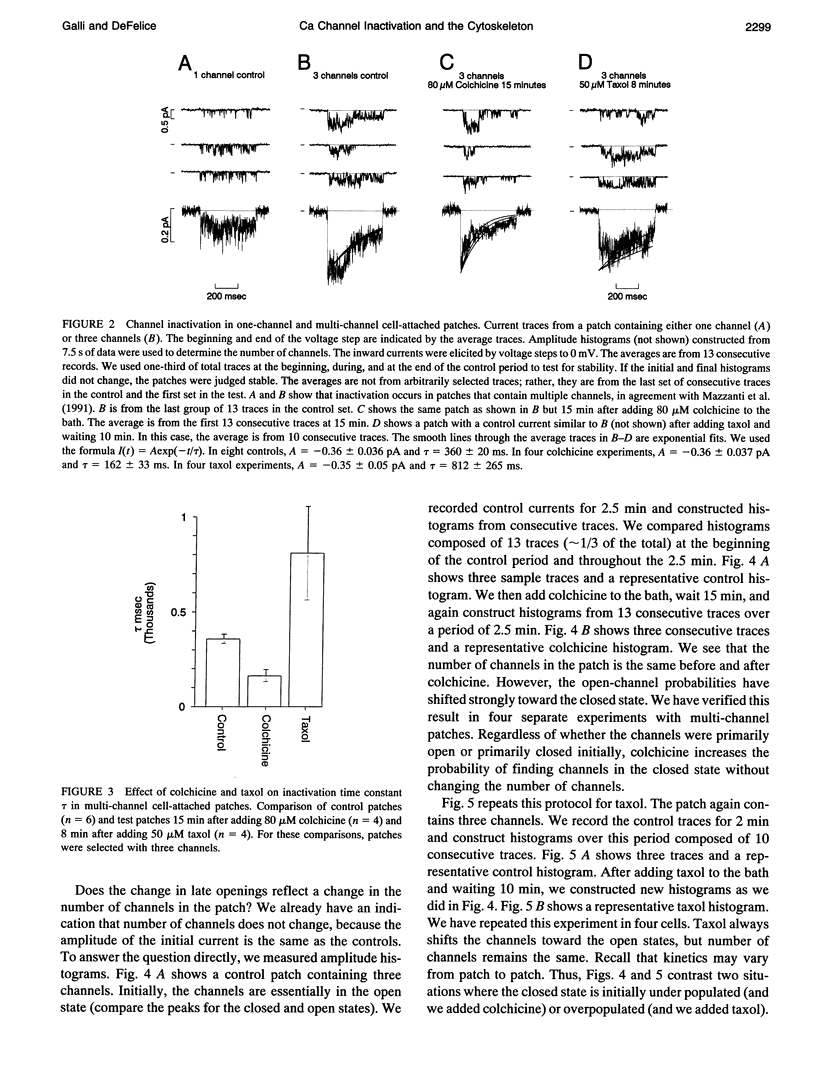
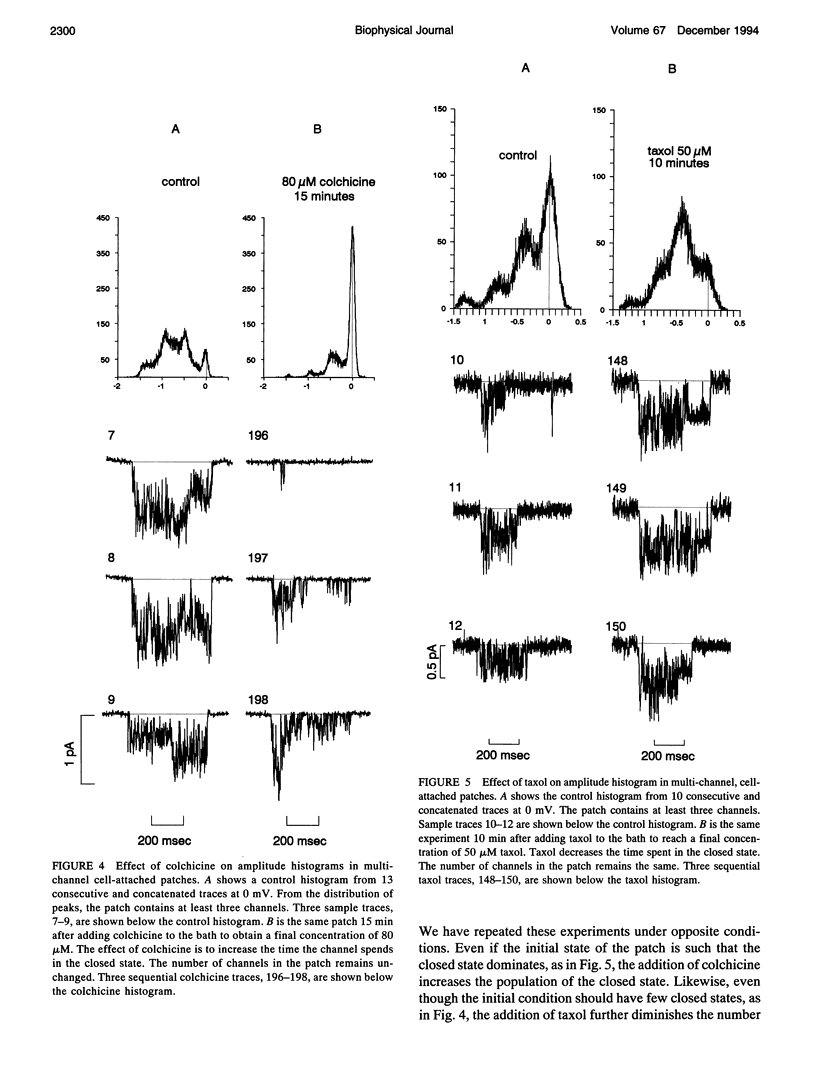
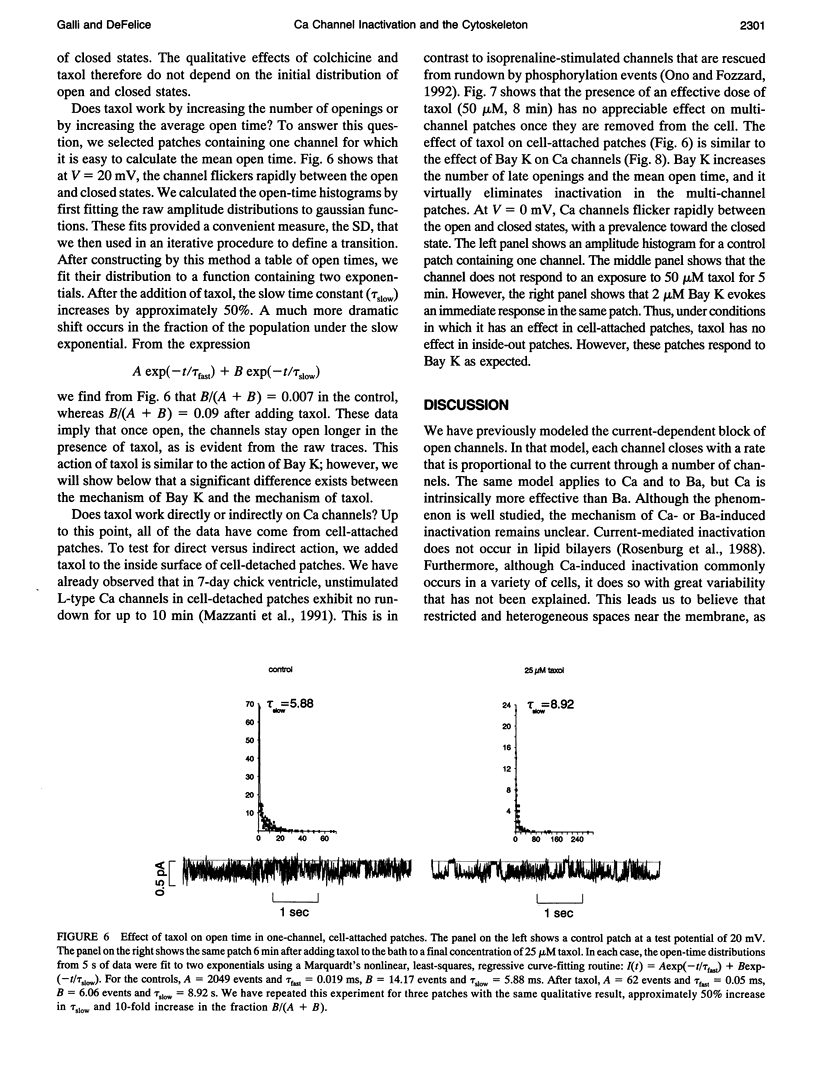
This article shows that colchicine and taxol strongly influence the kinetics of L-type Ca channels in intact cardiac cells, and it suggests a mechanism for this action. It is known that colchicine disassociates microtubules into tubulin, and that taxol stabilizes microtubules. We have found that colchicine increases the probability that Ca channels are in the closed state and that taxol increases the probability they are in the open state. Moreover, taxol lengthens the mean open time of Ca channels. In this regard, taxol is similar to Bay-K 8644; however, Bay K works on inside-out patches, but taxol does not. Neither colchicine nor taxol alters the number of Ca channels in a patch. We have quantified these results as follows. It is known that L-type channels in embryonic chick heart ventricle cells have voltage- and current-dependent inactivation. In 10 mM Ba, channel conductance is linear in the range -10 to 20 mV. The conductance is 12 +/- 1 pS, and the extrapolated reversal potential is 42 +/- 2 mV (n = 3). In cell-attached patches, inactivation depends on the number of channels. One channel (holding at -80 mV and stepping to 0 mV for 500 ms) shows virtually no inactivation. However, three channels inactivate with a time constant of 360 +/- 20 ms (n = 6). In similar patches, colchicine (80 microM for 15 min) decreases the inactivation time constant to 162 +/- 33 ms (n = 4) and taxol (50 microM for 10 min) virtually abolishes inactivation (time constant 812 +/- 265 ms (n = 4)). We suggest that colchicine and taxol affect Ca channels through their action on the cytoskeleton, which in turn regulates the effective concentration of inactivating ions near the mouths of channels. An alternate explanation is that free tubulin interacts directly with Ca channels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. L., Rossier M. F., Shcherbatko A. D., White R. E. Enzymatic gating of voltage-activated calcium channels. Ann N Y Acad Sci. 1991;635:26–34. doi: 10.1111/j.1749-6632.1991.tb36478.x. [DOI] [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Rios E. Nonlinear charge movement in mammalian cardiac ventricular cells. Components from Na and Ca channel gating. J Gen Physiol. 1989 Jul;94(1):65–93. doi: 10.1085/jgp.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Ionic channels and their regulation by G protein subunits. Annu Rev Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- Brum G., Osterrieder W., Trautwein W. Beta-adrenergic increase in the calcium conductance of cardiac myocytes studied with the patch clamp. Pflugers Arch. 1984 Jun;401(2):111–118. doi: 10.1007/BF00583870. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelice L. J. Molecular and biophysical view of the Ca channel: a hypothesis regarding oligomeric structure, channel clustering, and macroscopic current. J Membr Biol. 1993 May;133(3):191–202. doi: 10.1007/BF00232019. [DOI] [PubMed] [Google Scholar]
- Fujii S., Ayer R. K., Jr, DeHaan R. L. Development of the fast sodium current in early embryonic chick heart cells. J Membr Biol. 1988 Mar;101(3):209–223. doi: 10.1007/BF01872836. [DOI] [PubMed] [Google Scholar]
- Fukuda J., Kameyama M., Yamaguchi K. Breakdown of cytoskeletal filaments selectively reduces Na and Ca spikes in cultured mammal neurones. Nature. 1981 Nov 5;294(5836):82–85. doi: 10.1038/294082a0. [DOI] [PubMed] [Google Scholar]
- Galli A., Ferroni A., Bertollini L., Mazzanti M. Inactivation of single Ca2+ channels in rat sensory neurons by extracellular Ca2+. J Physiol. 1994 May 15;477(Pt 1):15–26. doi: 10.1113/jphysiol.1994.sp020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991 Dec;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Méry P. F., Fischmeister R., Szabo G. Sympathetic regulation of cardiac calcium current is due exclusively to cAMP-dependent phosphorylation. Nature. 1991 Jun 13;351(6327):573–576. doi: 10.1038/351573a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Fozzard H. A., January C. T. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol. 1989 May;256(5 Pt 2):H1478–H1492. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Mechanism of Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994 Jun;12(6):1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Submicroscopic Ca2+ diffusion mediates inhibitory coupling between individual Ca2+ channels. Neuron. 1992 Aug;9(2):197–207. doi: 10.1016/0896-6273(92)90159-b. [DOI] [PubMed] [Google Scholar]
- Johnson B. D., Byerly L. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+. Neuron. 1993 May;10(5):797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sperelakis N. Fast activation of cardiac Ca++ channel gating charge by the dihydropyridine agonist, BAY K 8644. Biophys J. 1990 Nov;58(5):1307–1311. doi: 10.1016/S0006-3495(90)82471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Aosaki T. Divalent cation dependent inactivation of the high-voltage-activated Ca-channel current in chick sensory neurons. Pflugers Arch. 1988 Jun;411(6):695–697. doi: 10.1007/BF00580869. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Inositol trisphosphate releases stored calcium to block voltage-dependent calcium channels in single smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):437–441. doi: 10.1007/BF00497770. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Liebmann J. E., Cook J. A., Lipschultz C., Teague D., Fisher J., Mitchell J. B. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br J Cancer. 1993 Dec;68(6):1104–1109. doi: 10.1038/bjc.1993.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Brown A. M. Single channel studies on inactivation of calcium currents. Science. 1984 Jul 27;225(4660):432–434. doi: 10.1126/science.6330896. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Ca channel gating during cardiac action potentials. Biophys J. 1990 Oct;58(4):1059–1065. doi: 10.1016/S0006-3495(90)82448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J., Liu Y. M. Gating of L-type Ca2+ channels in embryonic chick ventricle cells: dependence on voltage, current and channel density. J Physiol. 1991 Nov;443:307–334. doi: 10.1113/jphysiol.1991.sp018835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Fozzard H. A. Phosphorylation restores activity of L-type calcium channels after rundown in inside-out patches from rabbit cardiac cells. J Physiol. 1992 Aug;454:673–688. doi: 10.1113/jphysiol.1992.sp019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Fozzard H. A. Two phosphatase sites on the Ca2+ channel affecting different kinetic functions. J Physiol. 1993 Oct;470:73–84. doi: 10.1113/jphysiol.1993.sp019848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E., Wu L. T., Westcott D. Use of slow Ca2+ channel blockers to enhance inhibition by taxol of growth of drug-sensitive and -resistant Chinese hamster ovary cells. Cancer Treat Rep. 1986 Feb;70(2):275–278. [PubMed] [Google Scholar]
- Rasenick M. M., Stein P. J., Bitensky M. W. The regulatory subunit of adenylate cyclase interacts with cytoskeletal components. Nature. 1981 Dec 10;294(5841):560–562. doi: 10.1038/294560a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Risso S., DeFelice L. J. Ca channel kinetics during the spontaneous heart beat in embryonic chick ventricle cells. Biophys J. 1993 Sep;65(3):1006–1018. doi: 10.1016/S0006-3495(93)81147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. L., Hess P., Tsien R. W. Cardiac calcium channels in planar lipid bilayers. L-type channels and calcium-permeable channels open at negative membrane potentials. J Gen Physiol. 1988 Jul;92(1):27–54. doi: 10.1085/jgp.92.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokov R., Levis R., Shirokova N., Ríos E. Ca(2+)-dependent inactivation of cardiac L-type Ca2+ channels does not affect their voltage sensor. J Gen Physiol. 1993 Dec;102(6):1005–1030. doi: 10.1085/jgp.102.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A binding-site model for calcium channel inactivation that depends on calcium entry. Proc R Soc Lond B Biol Sci. 1982 Dec 22;217(1206):101–110. doi: 10.1098/rspb.1982.0097. [DOI] [PubMed] [Google Scholar]
- Wiernik P. H., Schwartz E. L., Einzig A., Strauman J. J., Lipton R. B., Dutcher J. P. Phase I trial of taxol given as a 24-hour infusion every 21 days: responses observed in metastatic melanoma. J Clin Oncol. 1987 Aug;5(8):1232–1239. doi: 10.1200/JCO.1987.5.8.1232. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Backx P. H., Imredy J. P. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990 Dec 21;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]



