Abstract
We have probed a cysteine residue that confers resistance to tetrodotoxin (TTX) block in heart Na channels, with membrane-impermeant, cysteine-specific, methanethiosulfonate (MTS) analogs. Covalent addition of a positively charged group to the cysteinyl sulfhydryl reduced pore conductance by 87%. The effect was selectively prevented by treatment with TTX, but not saxitoxin (STX). Addition of a negatively charged group selectively inhibited STX block without affecting TTX block. These results agree with models that place an exposed cysteinyl sulfhydryl in the TTX site adjacent to the mouth of the pore, but do not support the contention that STX and TTX are interchangeable. The surprising differences between the two toxins are consistent with the hypothesis that the toxin-receptor complex can assume different conformations when STX or TTX bound.
Full text
PDF

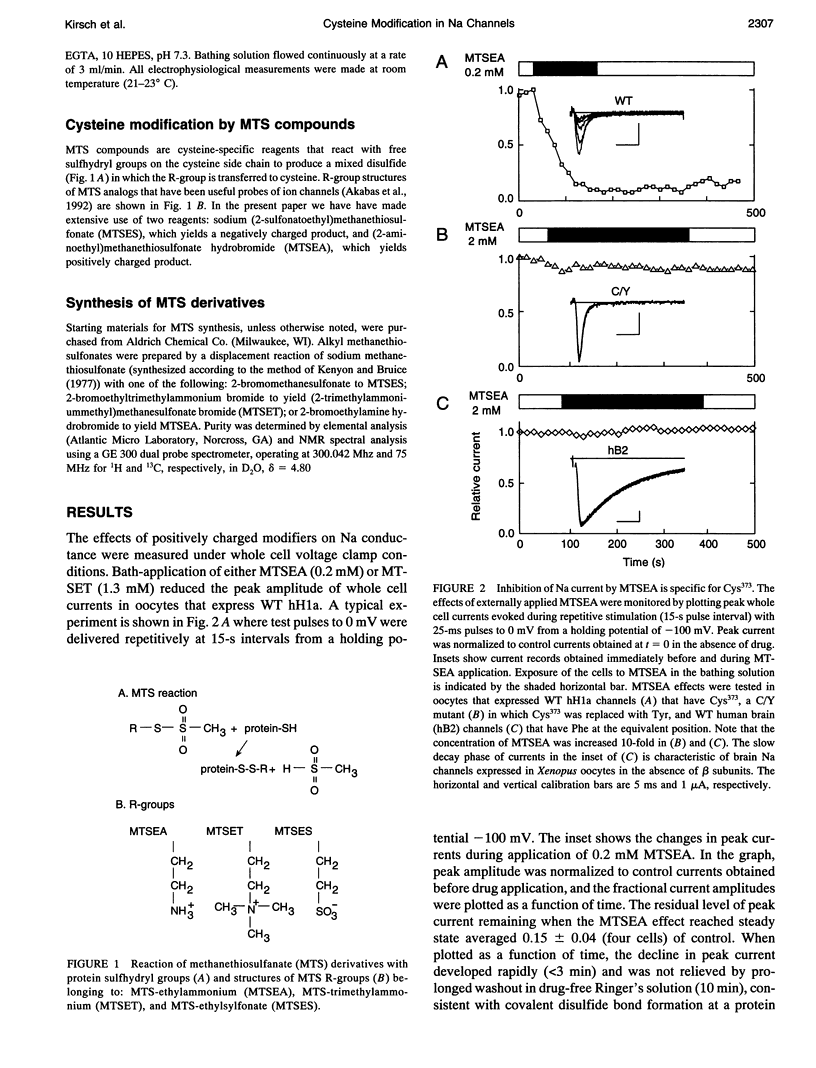
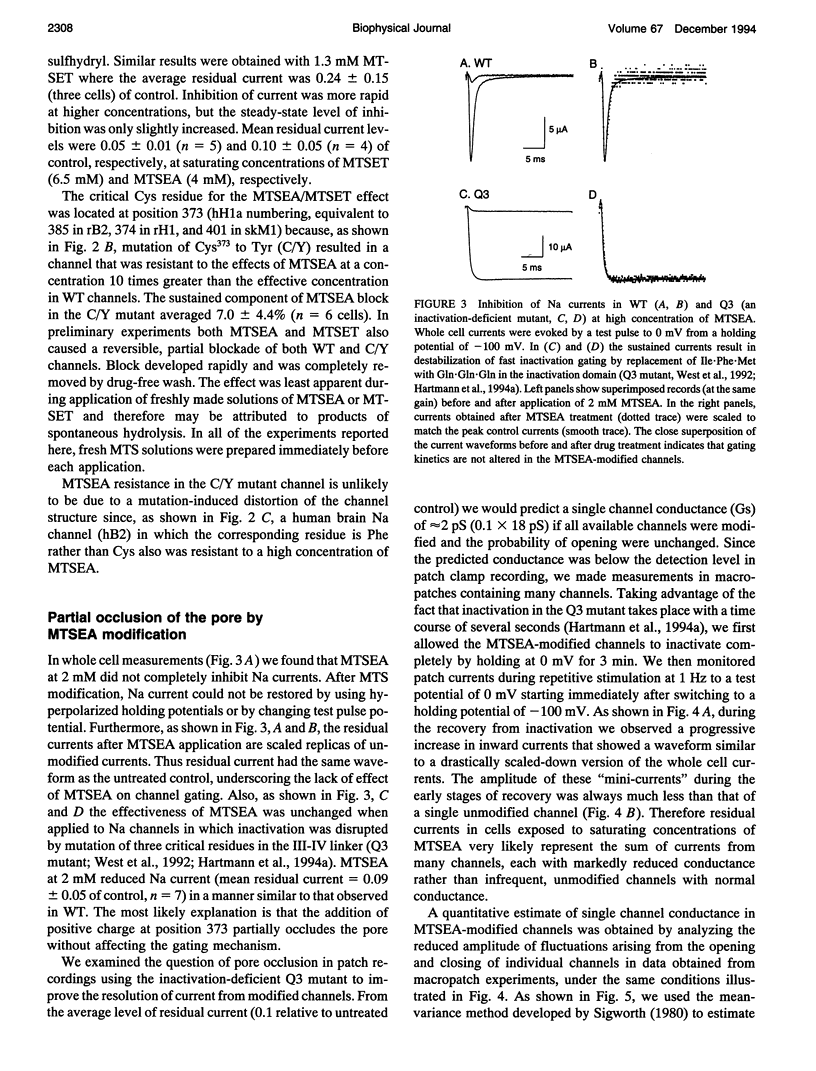
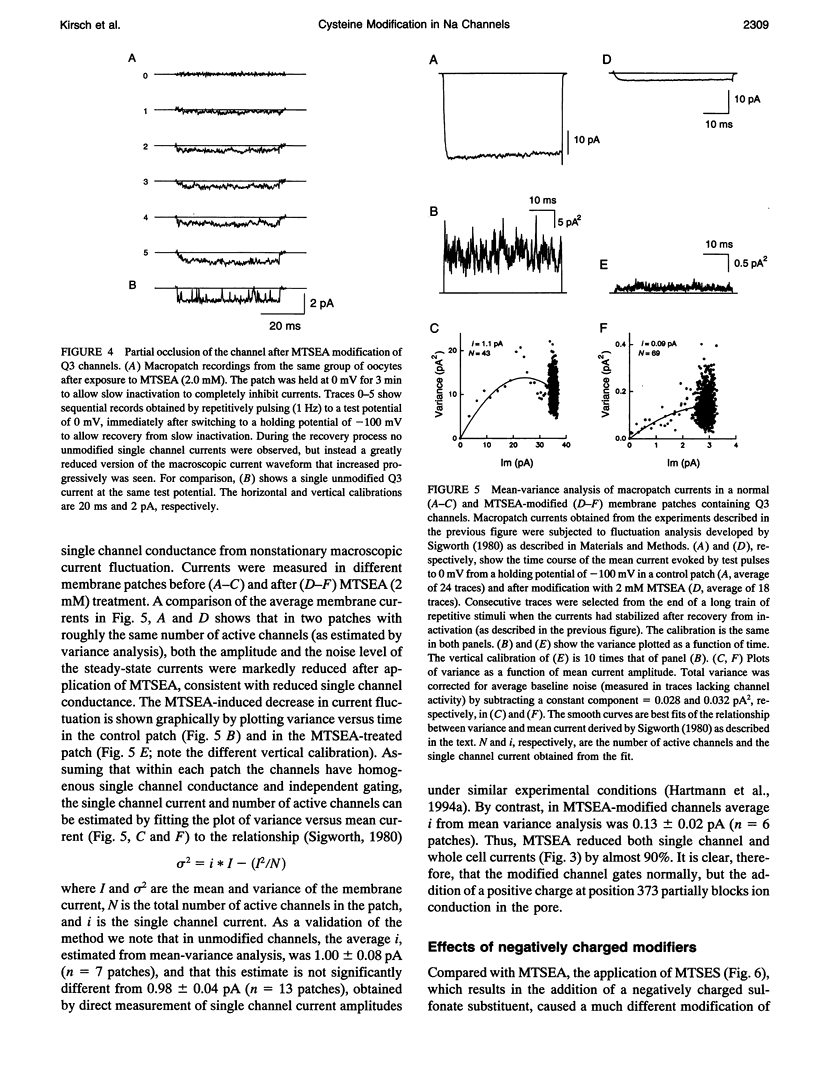
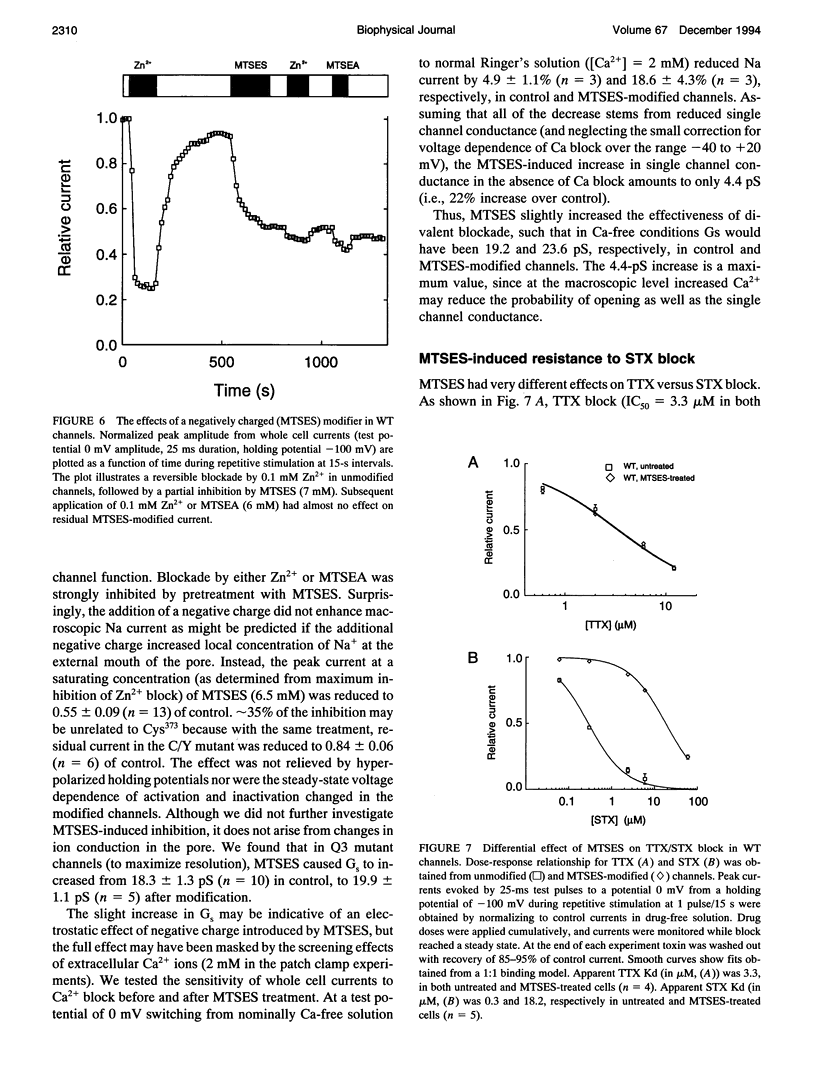
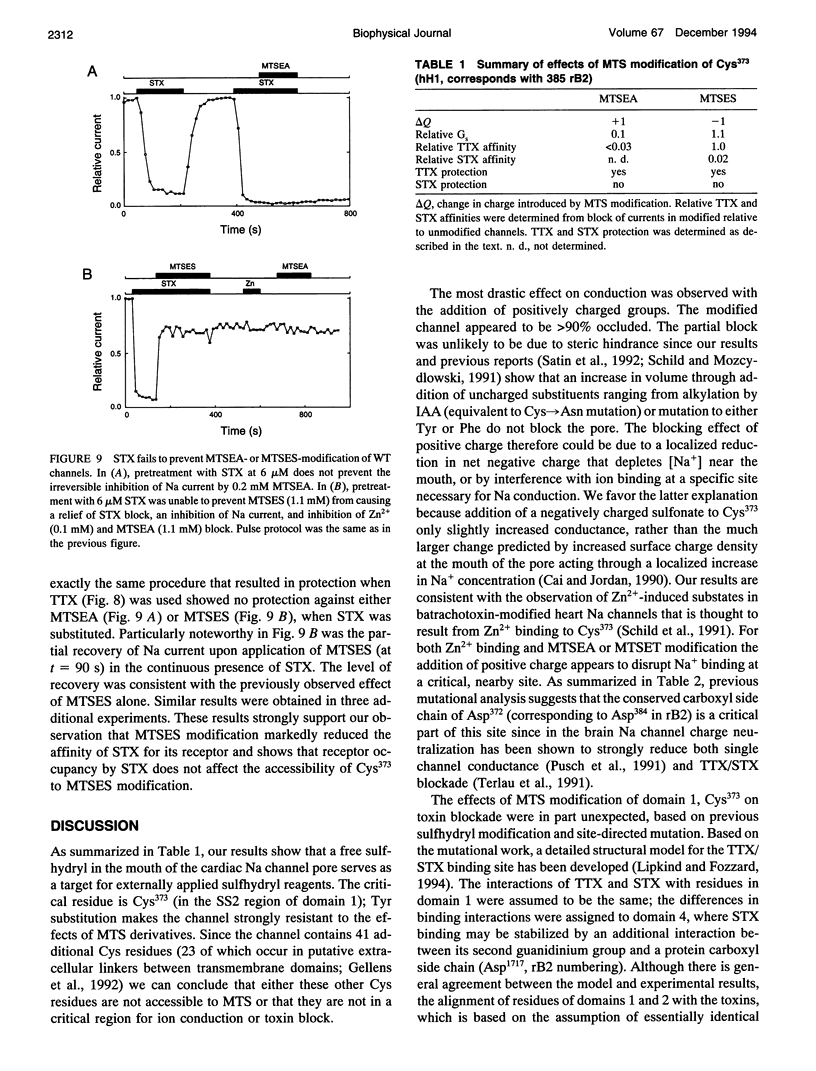



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akabas M. H., Stauffer D. A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992 Oct 9;258(5080):307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Backx P. H., Yue D. T., Lawrence J. H., Marban E., Tomaselli G. F. Molecular localization of an ion-binding site within the pore of mammalian sodium channels. Science. 1992 Jul 10;257(5067):248–251. doi: 10.1126/science.1321496. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Weigele J. B. Characteristics of saxitoxin binding to the sodium channel of sarcolemma isolated from rat skeletal muscle. J Physiol. 1979 Oct;295:383–396. doi: 10.1113/jphysiol.1979.sp012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay C. M., Strichartz G. R. Saxitoxin binding to sodium channels of rat skeletal muscles. J Physiol. 1980 Mar;300:89–103. doi: 10.1113/jphysiol.1980.sp013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M., Jordan P. C. How does vestibule surface charge affect ion conduction and toxin binding in a sodium channel? Biophys J. 1990 Apr;57(4):883–891. doi: 10.1016/S0006-3495(90)82608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabala L. D., Andersen O. S. Carbodiimide modification reduces the conductance and increases the tetrodotoxin sensitivity in batrachotoxin-modified sodium channels. Pflugers Arch. 1992 Jun;421(2-3):262–269. doi: 10.1007/BF00374836. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Bean B. P., Colatsky T. J., Tsien R. W. Tetrodotoxin block of sodium channels in rabbit Purkinje fibers. Interactions between toxin binding and channel gating. J Gen Physiol. 1981 Oct;78(4):383–411. doi: 10.1085/jgp.78.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. A., Barchi R. L. Voltage-dependent sodium channels. Int Rev Cytol. 1993;137C:55–103. [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Visentin S., Zaza A. Cadmium-induced blockade of the cardiac fast Na channels in calf Purkinje fibres. Proc R Soc Lond B Biol Sci. 1985 Feb 22;223(1233):475–484. doi: 10.1098/rspb.1985.0013. [DOI] [PubMed] [Google Scholar]
- Doyle D. D., Guo Y., Lustig S. L., Satin J., Rogart R. B., Fozzard H. A. Divalent cation competition with [3H]saxitoxin binding to tetrodotoxin-resistant and -sensitive sodium channels. A two-site structural model of ion/toxin interaction. J Gen Physiol. 1993 Feb;101(2):153–182. doi: 10.1085/jgp.101.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Gellens M. E., George A. L., Jr, Chen L. Q., Chahine M., Horn R., Barchi R. L., Kallen R. G. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W. N., Weiss L. B., Andersen O. S. Batrachotoxin-modified sodium channels in planar lipid bilayers. Characterization of saxitoxin- and tetrodotoxin-induced channel closures. J Gen Physiol. 1987 Jun;89(6):873–903. doi: 10.1085/jgp.89.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. T., Uehara A., Ravindran A., Bryant S. H., Hall S., Moczydlowski E. Kinetic basis for insensitivity to tetrodotoxin and saxitoxin in sodium channels of canine heart and denervated rat skeletal muscle. Biochemistry. 1987 Dec 1;26(24):7546–7556. doi: 10.1021/bi00398a003. [DOI] [PubMed] [Google Scholar]
- Hahin R., Strichartz G. Effects of deuterium oxide on the rate and dissociation constants for saxitoxin and tetrodotoxin action. Voltage-clamp studies on frog myelinated nerve. J Gen Physiol. 1981 Aug;78(2):113–139. doi: 10.1085/jgp.78.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N. L., Radke H. K., Talukder G., Ffrench-Mullen J. M. Zinc modulates transient outward current gating in hippocampal neurons. Receptors Channels. 1993;1(2):153–163. [PubMed] [Google Scholar]
- Hartmann H. A., Tiedeman A. A., Chen S. F., Brown A. M., Kirsch G. E. Effects of III-IV linker mutations on human heart Na+ channel inactivation gating. Circ Res. 1994 Jul;75(1):114–122. doi: 10.1161/01.res.75.1.114. [DOI] [PubMed] [Google Scholar]
- Heinemann S. H., Conti F. Nonstationary noise analysis and application to patch clamp recordings. Methods Enzymol. 1992;207:131–148. doi: 10.1016/0076-6879(92)07009-d. [DOI] [PubMed] [Google Scholar]
- Heinemann S. H., Terlau H., Imoto K. Molecular basis for pharmacological differences between brain and cardiac sodium channels. Pflugers Arch. 1992 Oct;422(1):90–92. doi: 10.1007/BF00381519. [DOI] [PubMed] [Google Scholar]
- Henderson R., Ritchie J. M., Strichartz G. R. Evidence that tetrodotoxin and saxitoxin act at a metal cation binding site in the sodium channels of nerve membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3936–3940. doi: 10.1073/pnas.71.10.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. C., Peng Y. W., Yau K. W. Zinc modulation of a transient potassium current and histochemical localization of the metal in neurons of the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11806–11810. doi: 10.1073/pnas.90.24.11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y. Structure-activity relations of tetrodotoxin, saxitoxin, and analogues. Ann N Y Acad Sci. 1986;479:52–67. doi: 10.1111/j.1749-6632.1986.tb15561.x. [DOI] [PubMed] [Google Scholar]
- Kenyon G. L., Bruice T. W. Novel sulfhydryl reagents. Methods Enzymol. 1977;47:407–430. doi: 10.1016/0076-6879(77)47042-3. [DOI] [PubMed] [Google Scholar]
- Lipkind G. M., Fozzard H. A. A structural model of the tetrodotoxin and saxitoxin binding site of the Na+ channel. Biophys J. 1994 Jan;66(1):1–13. doi: 10.1016/S0006-3495(94)80746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Hall S., Garber S. S., Strichartz G. S., Miller C. Voltage-dependent blockade of muscle Na+ channels by guanidinium toxins. J Gen Physiol. 1984 Nov;84(5):687–704. doi: 10.1085/jgp.84.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M., Noda M., Stühmer W., Numa S., Conti F. Single point mutations of the sodium channel drastically reduce the pore permeability without preventing its gating. Eur Biophys J. 1991;20(3):127–133. doi: 10.1007/BF01561134. [DOI] [PubMed] [Google Scholar]
- Ravindran A., Schild L., Moczydlowski E. Divalent cation selectivity for external block of voltage-dependent Na+ channels prolonged by batrachotoxin. Zn2+ induces discrete substates in cardiac Na+ channels. J Gen Physiol. 1991 Jan;97(1):89–115. doi: 10.1085/jgp.97.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogart R. B. High-STX-affinity vs. low-STX-affinity Na+ channel subtypes in nerve, heart, and skeletal muscle. Ann N Y Acad Sci. 1986;479:402–430. doi: 10.1111/j.1749-6632.1986.tb15585.x. [DOI] [PubMed] [Google Scholar]
- Satin J., Kyle J. W., Chen M., Bell P., Cribbs L. L., Fozzard H. A., Rogart R. B. A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science. 1992 May 22;256(5060):1202–1205. doi: 10.1126/science.256.5060.1202. [DOI] [PubMed] [Google Scholar]
- Schild L., Moczydlowski E. Competitive binding interaction between Zn2+ and saxitoxin in cardiac Na+ channels. Evidence for a sulfhydryl group in the Zn2+/saxitoxin binding site. Biophys J. 1991 Mar;59(3):523–537. doi: 10.1016/S0006-3495(91)82269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L., Ravindran A., Moczydlowski E. Zn2(+)-induced subconductance events in cardiac Na+ channels prolonged by batrachotoxin. Current-voltage behavior and single-channel kinetics. J Gen Physiol. 1991 Jan;97(1):117–142. doi: 10.1085/jgp.97.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y. Chemistry and biochemistry of saxitoxin analogues and tetrodotoxin. Ann N Y Acad Sci. 1986;479:24–31. doi: 10.1111/j.1749-6632.1986.tb15558.x. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor F. J., McHugh E., Catterall W. A. Stabilization of a sodium channel state with high affinity for saxitoxin by intramolecular cross-linking. Evidence for allosteric effects of saxitoxin binding. Biochemistry. 1988 Apr 5;27(7):2389–2397. doi: 10.1021/bi00407a021. [DOI] [PubMed] [Google Scholar]
- Terlau H., Heinemann S. H., Stühmer W., Pusch M., Conti F., Imoto K., Numa S. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 1991 Nov 18;293(1-2):93–96. doi: 10.1016/0014-5793(91)81159-6. [DOI] [PubMed] [Google Scholar]
- West J. W., Patton D. E., Scheuer T., Wang Y., Goldin A. L., Catterall W. A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. A., Alonso A., Kay A. R. A heart-like Na+ current in the medial entorhinal cortex. Neuron. 1993 Dec;11(6):1037–1047. doi: 10.1016/0896-6273(93)90217-f. [DOI] [PubMed] [Google Scholar]
- Yang L., Kao C. Y. Actions of chiriquitoxin on frog skeletal muscle fibers and implications for the tetrodotoxin/saxitoxin receptor. J Gen Physiol. 1992 Oct;100(4):609–622. doi: 10.1085/jgp.100.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarowsky P. J., Krueger B. K., Olson C. E., Clevinger E. C., Koos R. D. Brain and heart sodium channel subtype mRNA expression in rat cerebral cortex. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9453–9457. doi: 10.1073/pnas.88.21.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]