Abstract
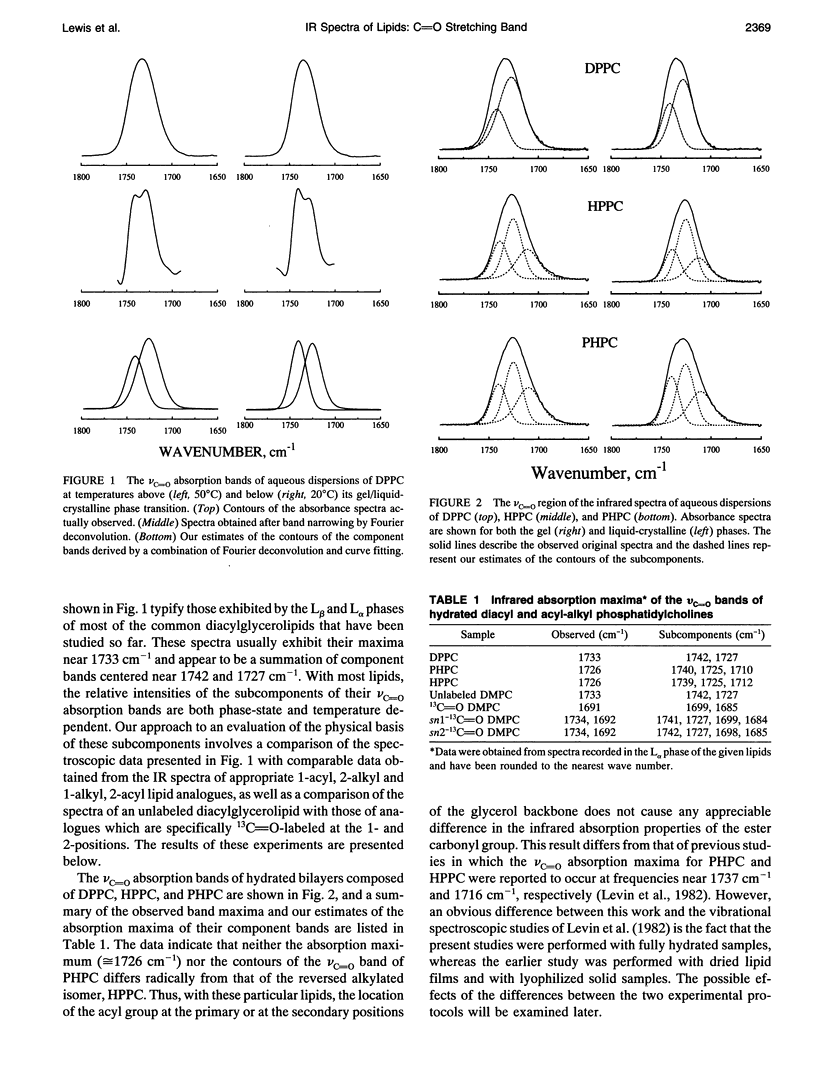
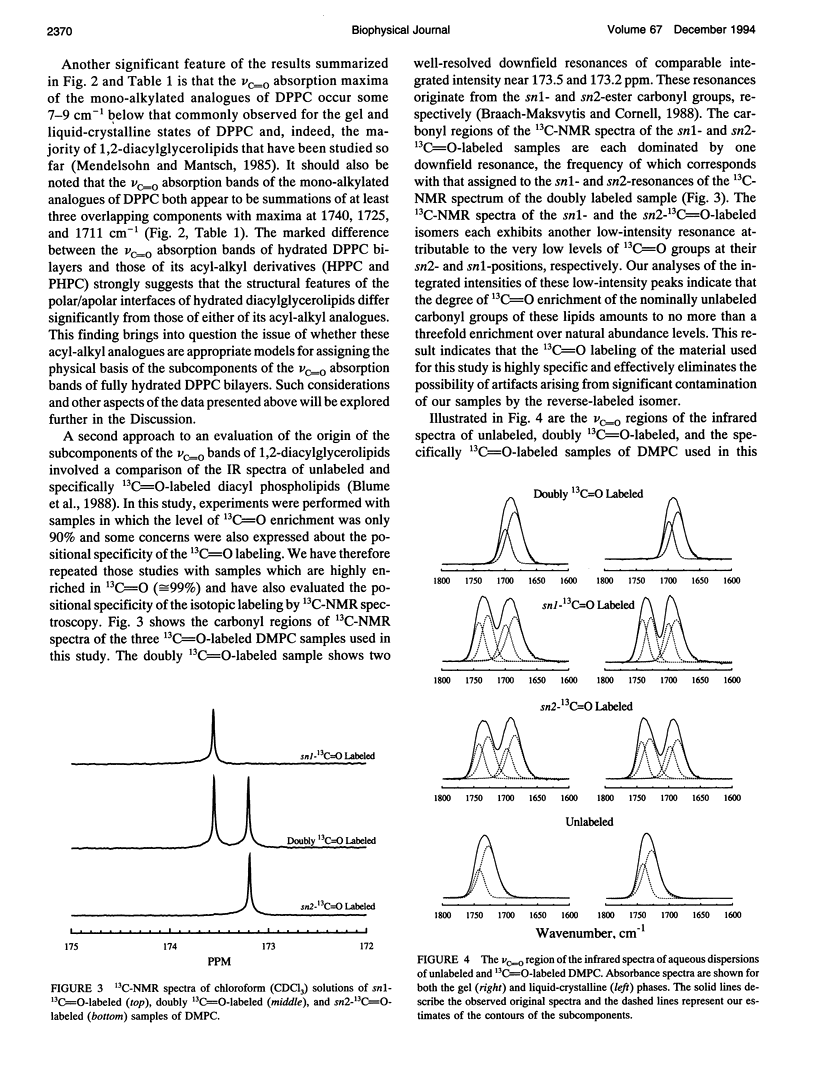
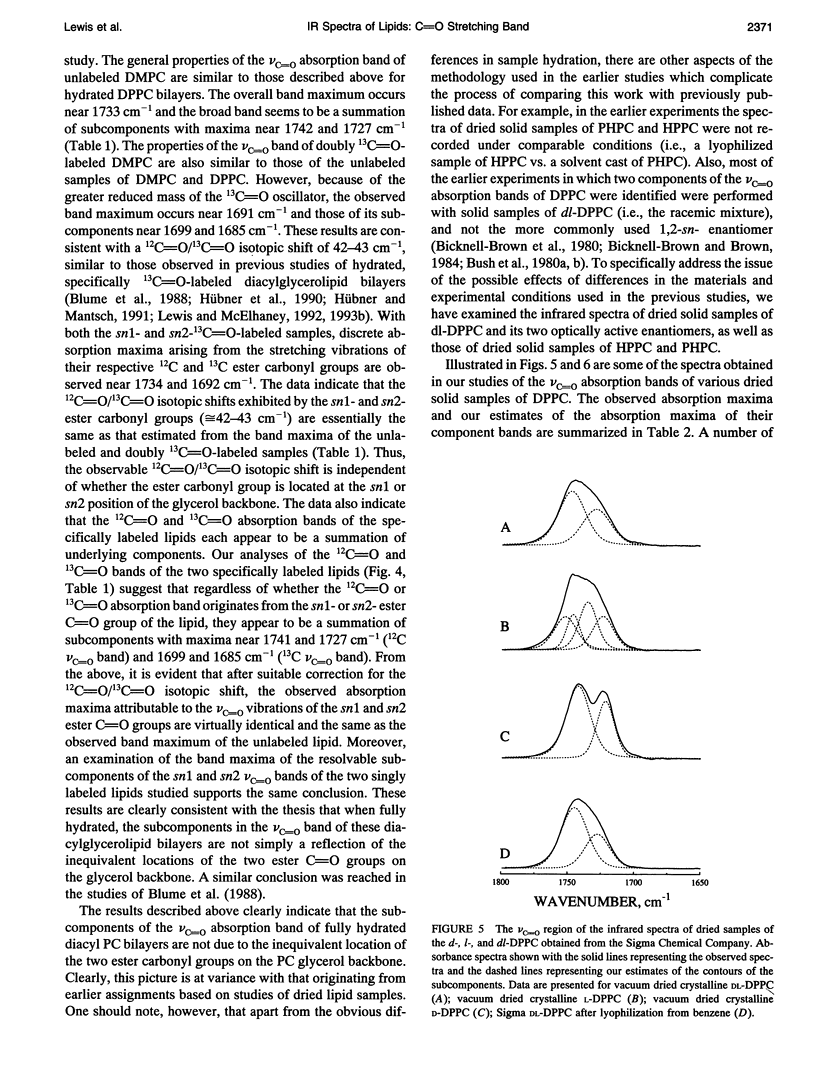
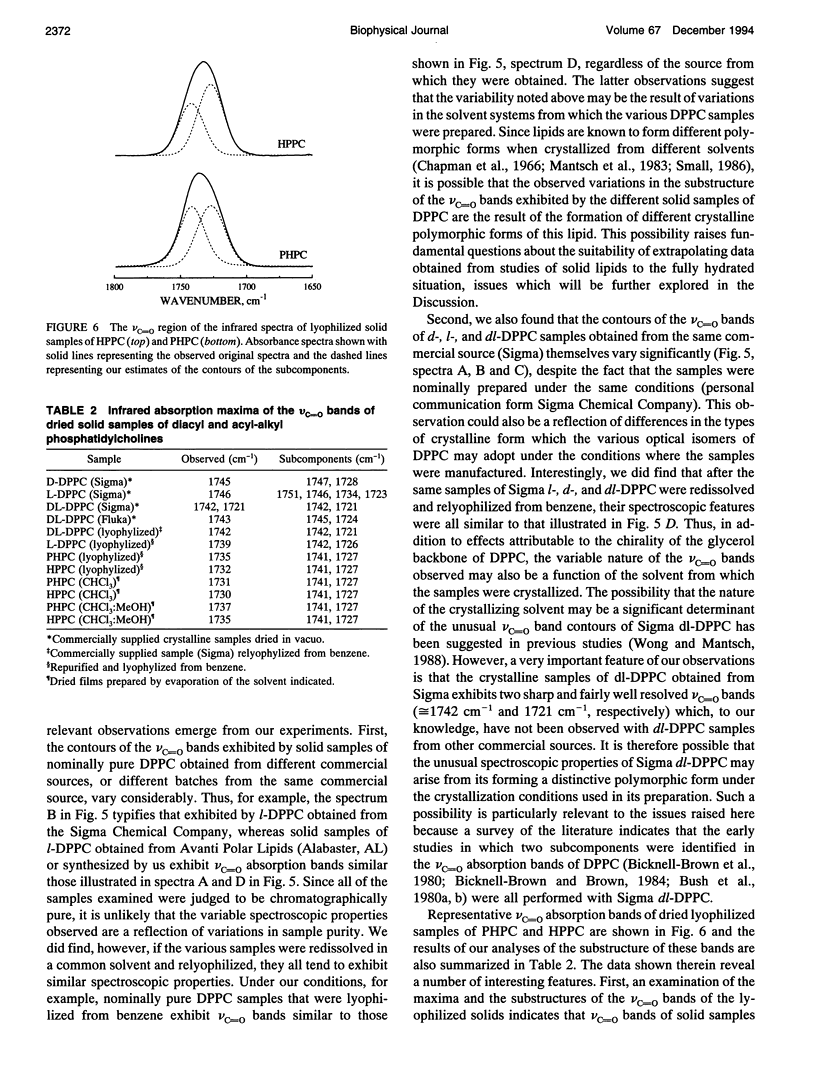
Previous vibrational spectroscopic studies of solid acyl-alkyl and diacyl phosphatidylcholines suggested that the sn1- and sn2-carbonyl stretching modes of 1,2-diacylglycerolipids have different absorption maxima. To address the assignment of sn1- and sn2-carbonyl stretching modes of hydrated 1,2-diacylglycerolipids, aqueous dispersions of 1-palmitoyl-2-hexadecyl phosphatidylcholine (PHPC), 1-hexadecyl-2-palmitoyl phosphatidylcholine (HPPC), 1,2-dipalmitoylphosphatidylcholine (DPPC), as well as hydrated samples of unlabeled, sn1-13C=O-labeled, sn2-13C=O-labeled, and doubly 13C=O-labeled dimyristoylphosphatidylcholine (DMPC) were examined by Fourier transform infrared spectroscopy. The ester carbonyl stretching (nu C=O) bands of HPPC and PHPC each exhibit maxima near 1726 cm-1 and appear to be a summation of three subcomponents with maxima near 1740 cm-1, 1725 and 1705-1711 cm-1. In contrast, the nu C=O band of DPPC exhibits its maximum near 1733 cm-1 and appears to be a summation of two components centered near 1742 and 1727 cm-1. Thus the ester carbonyl group of the acyl-alkyl PCs appears to reside in a more polar environment than the ester carbonyl groups of their diacyl analogue. This observation implies that the polar/apolar interfaces of hydrated bilayers formed by PHPC and by HPPC are significantly different from that of DPPC and raises the question of whether the acyl-alkyl PCs are suitable models of their diacyl analogue. The absorption maximum of the nu C=O band of the doubly 13C=O-labeled DMPC occurs near 1691 cm-1 and those of its subcomponents occur near 1699 and 1685 cm-1. These frequencies are consistent with a 12C=0/13C0 'isotopic shift' of 42-43cm-1. snl - and snY2-13C0O-labeled DMPC each exhibit well resolved 12C and 13C vc-0 bands with absorption maxima near 1734 and 1692 cm-1, respectively. With both specifically 13C=O-labeled lipids, the 12C and 13C vo bands each seem to be a summation of subcomponents with absorption maxima near 1742 and 1727 cm-1 (12C vc=o) and 1699 and 1685 cm-1 (13C VC_o),regardless of whether the 13C=O-labeled fatty acyl chain is esterified at the snl - or sn2- positions of the glycerol backbone.We conclude that in hydrated 1,2-diacyl PC bilayers, the patterns of infrared absorption exhibited by ester carbonyl groups located at the primary and secondary positions of the glycerol backbone are similar. Also, the resolvable subcomponents of their v0 bands are each a summation of comparable contributions from both ester carbonyl groups and therefore cannot be attributed to the inequivalent locations of the two ester carbonyl groups. This result differs from that of the vibrational spectroscopic studies alluded to above and raises the question of whether data obtained in studies of dry (or poorly hydrated) lipids are applicable to fully hydrated lipid bilayers. To address questions of why the results of the two studies differ, we have also examined the vc=o bands of solid samples of DPPC, HPPC, and PHPC. We find that the vc-0 bands of all solid lipids studied differ from those of the hydrated samples. Moreover, with solid lipids the vc=o bands vary with the enantiomeric configuration,enantiomeric purity and thermal thermal history as well as with the way in which the sample was prepared. Also, although the vc=o bands of solid HPPC and PHPC vary significantly with sample preparation methodology, samples of PHPC and HPPCprepared by the same method exhibit very similar vC-0 absorption bands. We conclude as far as the organization of lipid polar/apolar interfaces is concerned, solid lipids are not good models of hydrated lipid bilayers and suggest that this may be largely responsible for the different conclusions drawn in this work and in previously published studies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C = O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988 Oct 18;27(21):8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- Braach-Maksvytis V. L., Cornell B. A. Chemical shift anisotropies obtained from aligned egg yolk phosphatidylcholine by solid-state 13C nuclear magnetic resonance. Biophys J. 1988 May;53(5):839–843. doi: 10.1016/S0006-3495(88)83163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush S. F., Adams R. G., Levin I. W. Structural reorganizations in lipid bilayer systems: effect of hydration and sterol addition on Raman spectra of dipalmitoylphosphatidylcholine multilayers. Biochemistry. 1980 Sep 16;19(19):4429–4436. doi: 10.1021/bi00560a008. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Sundell S. Preferred conformation and dynamics of the glycerol backbone in phospholipids. An NMR and X-ray single-crystal analysis. Biochemistry. 1988 Dec 27;27(26):9166–9174. doi: 10.1021/bi00426a014. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. B., Mason R., Thomas K. M., Shipley G. G. Structural chemistry of 1,2 dilauroyl-DL-phosphatidylethanolamine: molecular conformation and intermolecular packing of phospholipids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3036–3040. doi: 10.1073/pnas.71.8.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner W., Mantsch H. H. Orientation of specifically 13C=O labeled phosphatidylcholine multilayers from polarized attenuated total reflection FT-IR spectroscopy. Biophys J. 1991 Jun;59(6):1261–1272. doi: 10.1016/S0006-3495(91)82341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Calorimetric and spectroscopic studies of the polymorphic phase behavior of a homologous series of n-saturated 1,2-diacyl phosphatidylethanolamines. Biophys J. 1993 Apr;64(4):1081–1096. doi: 10.1016/S0006-3495(93)81474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Structures of the subgel phases of n-saturated diacyl phosphatidylcholine bilayers: FTIR spectroscopic studies of 13C = O and 2H labeled lipids. Biophys J. 1992 Jan;61(1):63–77. doi: 10.1016/S0006-3495(92)81816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Studies of mixed-chain diacyl phosphatidylcholines with highly asymmetric acyl chains: a Fourier transform infrared spectroscopic study of interfacial hydration and hydrocarbon chain packing in the mixed interdigitated gel phase. Biophys J. 1993 Nov;65(5):1866–1877. doi: 10.1016/S0006-3495(93)81251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 1. Differential scanning calorimetric studies. Biochemistry. 1985 May 7;24(10):2431–2439. doi: 10.1021/bi00331a007. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Hsi S. C., Butler K. W., Cameron D. G. Studies on the thermotropic behavior of aqueous phosphatidylethanolamines. Biochim Biophys Acta. 1983 Mar 9;728(3):325–330. doi: 10.1016/0005-2736(83)90502-3. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Madec C., Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 2. Infrared and 31P NMR spectroscopic studies. Biochemistry. 1985 May 7;24(10):2440–2446. doi: 10.1021/bi00331a008. [DOI] [PubMed] [Google Scholar]
- Mushayakarara E. C., Wong P. T., Mantsch H. H. Detection by high pressure infrared spectrometry of hydrogen-bonding between water and triacetyl glycerol. Biochem Biophys Res Commun. 1986 Jan 14;134(1):140–145. doi: 10.1016/0006-291x(86)90538-3. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]


