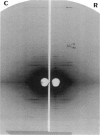
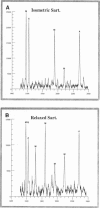
Abstract
We have used a small angle scattering system assembled on the high flux multipole wiggler beam line at CHESS (Cornell) to make very accurate spacing measurements of certain meridional and layer-line reflections from contracting muscles. During isometric contraction, the actin 27.3 A reflection increases in spacing from its resting value by approximately 0.3%, and other actin reflections, including the 59 and 51 A off-meridional reflections, show corresponding changes in spacing. When tension is augmented or diminished by applying moderate speed length changes to a contracting muscle, changes in spacing in the range of 0.19-0.24% (when scaled to full isometric tension) can be seen. The larger difference between the resting and isometric spacings suggests either nonlinearity at low tension levels or the presence of a component related to activation itself. Myosin filaments also show similar increases in axial period during slow stretch, in addition to the well known larger change associated with activation. An actin spacing change of 0.25-0.3% can also be measured during a 2 ms time frame immediately after a quick release, showing that the elastic behavior is rapid. These observations of filament extensions totaling 2-3 nm per half-sarcomere may necessitate some significant revision of the interpretation of a number of mechanical experiments in muscle, in which it has usually been assumed that virtually all of the elasticity resides in the cross-bridges.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elliott G. F., Lowy J., Millman B. M. X-ray diffraction from living striated muscle during contraction. Nature. 1965 Jun 26;206(991):1357–1358. doi: 10.1038/2061357a0. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes F., Mickey B., Nettleton J., Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol. 1993 Feb;120(4):923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W., Holmes K. C. Constancy of axial spacings in frog sartorius muscle during contraction. Nature. 1965 Jun 26;206(991):1358–1358. doi: 10.1038/2061358a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Kress M., Bordas J., Koch M. H. Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J Mol Biol. 1982 Jul 15;158(4):637–684. doi: 10.1016/0022-2836(82)90253-4. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Simmons R. M., Faruqi A. R., Kress M., Bordas J., Koch M. H. Changes in the X-ray reflections from contracting muscle during rapid mechanical transients and their structural implications. J Mol Biol. 1983 Sep 15;169(2):469–506. doi: 10.1016/s0022-2836(83)80062-x. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Oosawa F. The flexibility of F-actin. Biophys Chem. 1980 Jun;11(3-4):443–446. doi: 10.1016/0301-4622(80)87021-9. [DOI] [PubMed] [Google Scholar]
- Padrón R., Craig R. Disorder induced in nonoverlap myosin cross-bridges by loss of adenosine triphosphate. Biophys J. 1989 Nov;56(5):927–933. doi: 10.1016/S0006-3495(89)82738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström M., Squire J. M. Fine structure of the A-band in cryo-sections. The structure of the A-band of human skeletal muscle fibres from ultra-thin cryo-sections negatively stained. J Mol Biol. 1977 Jan 5;109(1):49–68. doi: 10.1016/s0022-2836(77)80045-4. [DOI] [PubMed] [Google Scholar]