Abstract
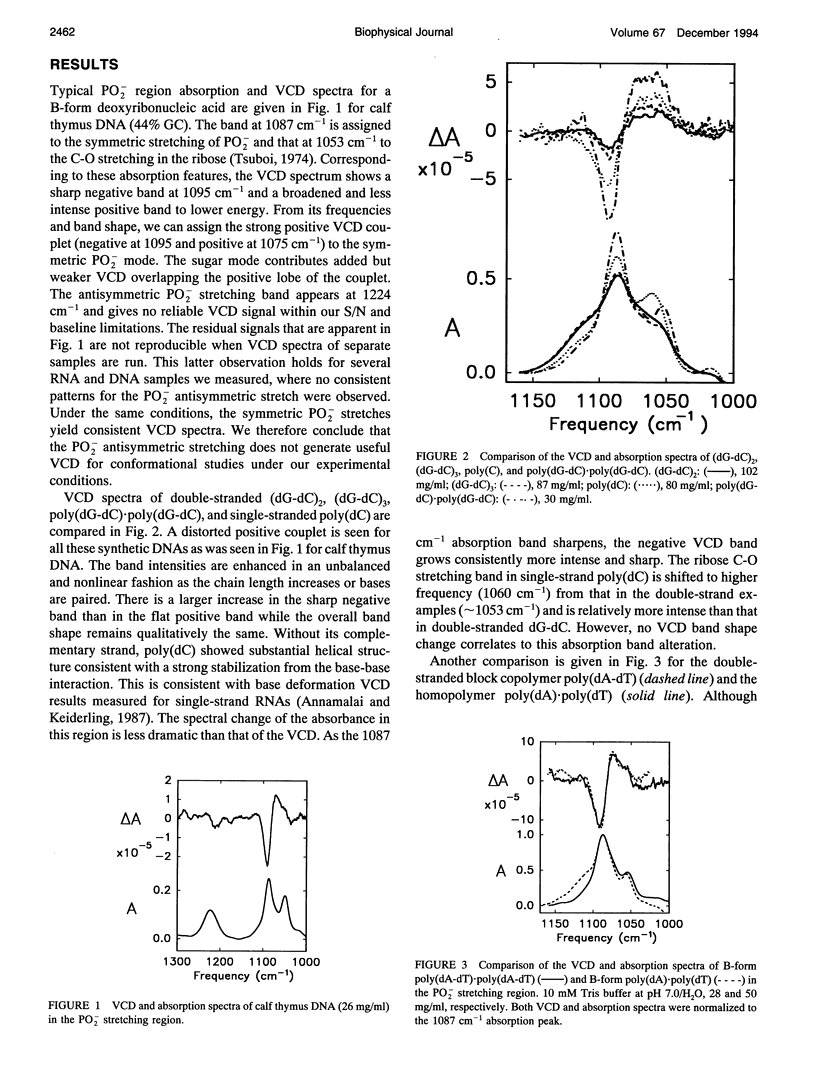
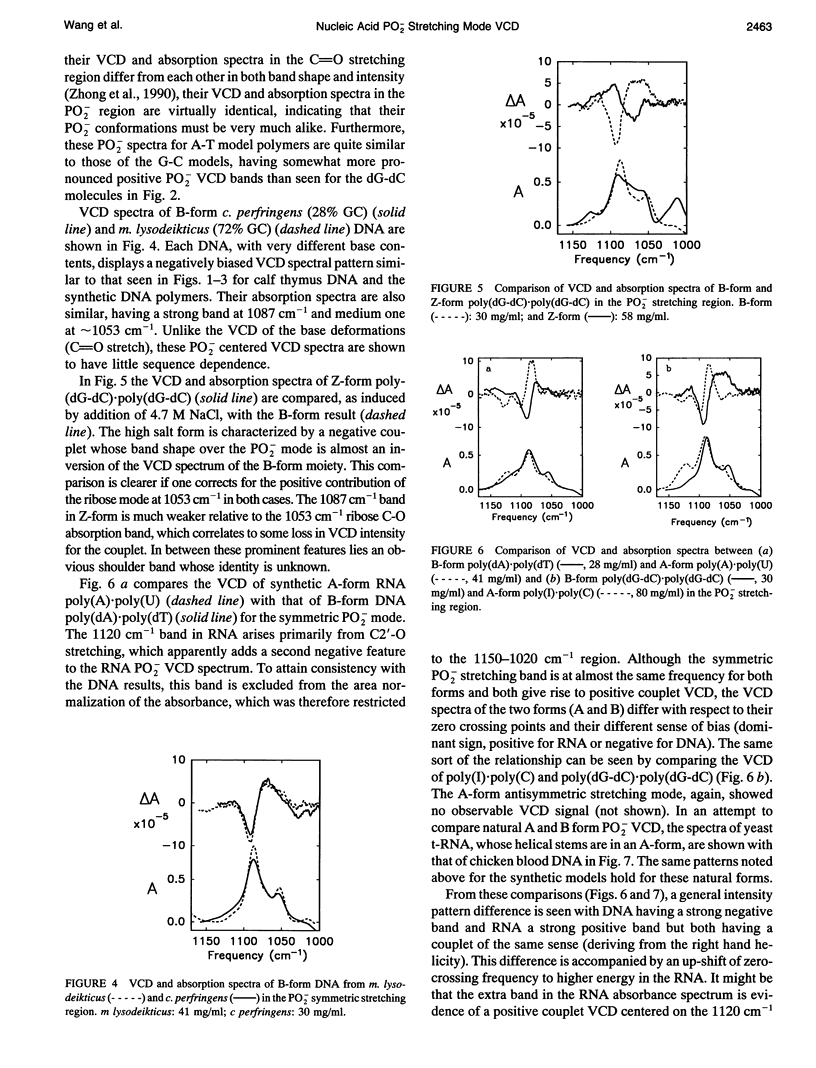
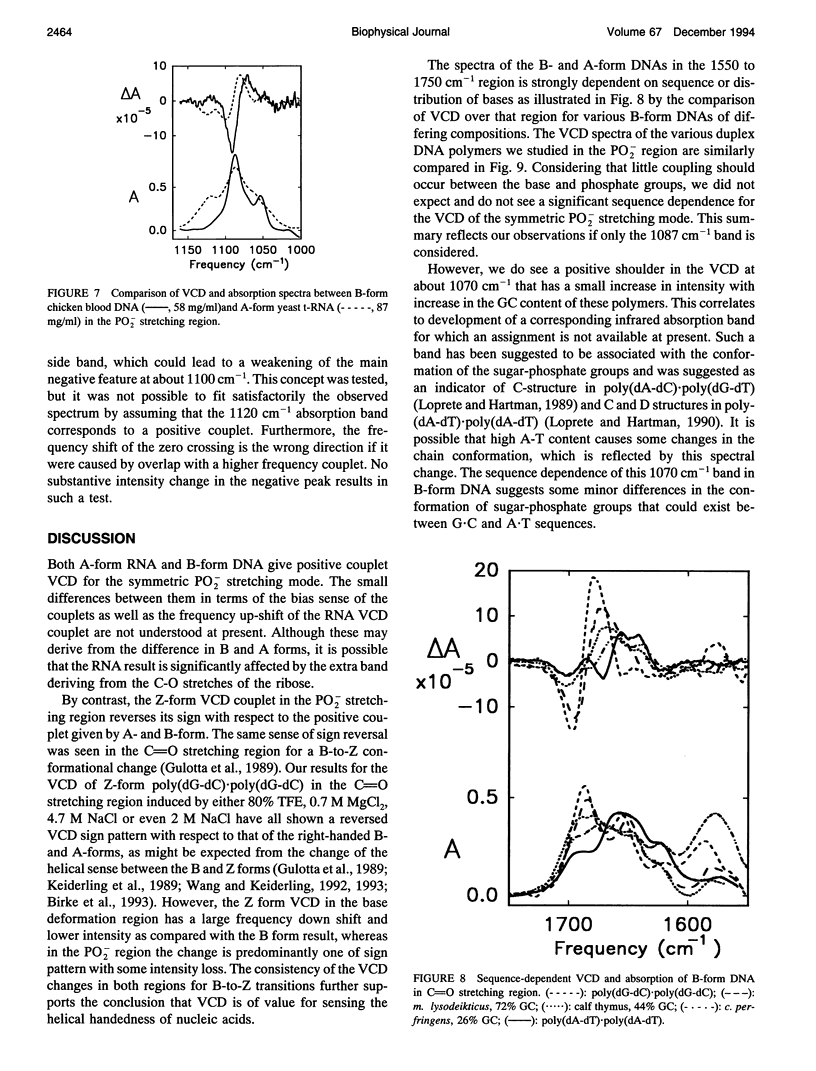
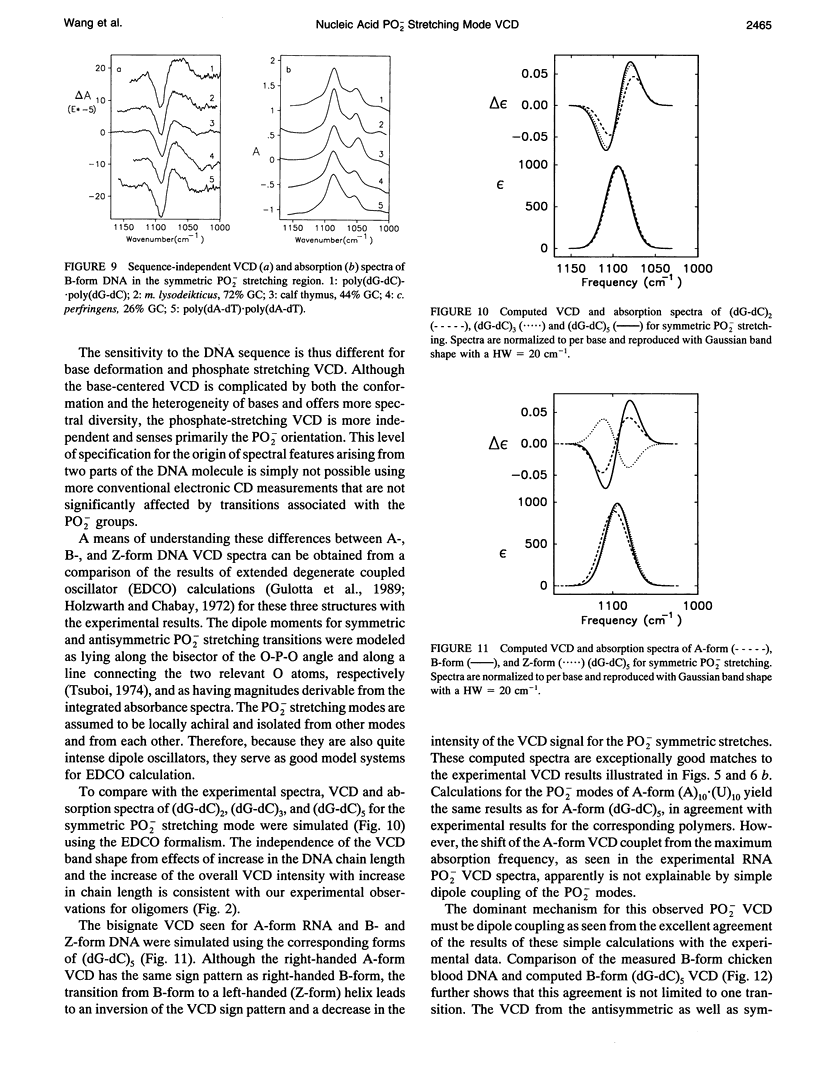
Vibrational circular dichroism (VCD) spectra were measured for H2O solutions of several natural and model DNAs (single and double strands, oligomers and polymers) in the B-form, poly(dG-dC)-poly(dG-dC) in the Z-form, and various duplex RNAs in an A-form over the PO2-stretching region. Only the symmetric PO2 stretch at approximately 1075 cm-1 yields a significant intensity VCD signal. Differences of the PO2-stretching VCD spectra found for these conformational types are consistent with the spectral changes seen in the base region, but no sequence dependence was seen in contrast to VCD for base modes. The B to Z transition is accompanied by an inversion of the PO2- VCD spectra, which is characteristic of the change in the helical sense of the nucleic acid backbone. A-RNAs give rise to the same sense of couplet VCD as do B-DNAs but have a somewhat different shape because of overlapping ribose modes. These PO2- VCD spectral characteristics have been successfully modeled using simple dipole coupling calculations. The invariability of the symmetric PO2- stretching mode VCD spectra to the base sequence as opposed to that found for the C = O stretching and base deformation modes is evidence that this mode will provide a stable indication of the DNA helical sense.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291X(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Birke S. S., Moses M., Kagalovsky B., Jano D., Gulotta M., Diem M. Infrared CD of deoxy oligonucleotides. Conformational studies of 5'd(GCGC)3', 5'd(CGCG)3', 5'd(CCGG)3', and 5'd(GGCC)3' in low and high salt aqueous solution. Biophys J. 1993 Sep;65(3):1262–1271. doi: 10.1016/S0006-3495(93)81176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulotta M., Goss D. J., Diem M. IR vibrational CD in model deoxyoligonucleotides: observation of the B----Z phase transition and extended coupled oscillator intensity calculations. Biopolymers. 1989 Dec;28(12):2047–2058. doi: 10.1002/bip.360281202. [DOI] [PubMed] [Google Scholar]
- Loprete D. M., Hartman K. A. Existence of the C structure in poly(dA-dC).poly(dG-dT). J Biomol Struct Dyn. 1989 Oct;7(2):347–362. doi: 10.1080/07391102.1989.10507777. [DOI] [PubMed] [Google Scholar]
- Pilet J., Brahms J. Dependence of B-A conformational change in DNA on base composition. Nat New Biol. 1972 Mar 29;236(65):99–100. doi: 10.1038/newbio236099a0. [DOI] [PubMed] [Google Scholar]
- Wang L., Keiderling T. A. Helical nature of poly(dI-dC).poly(dI-dC). Vibrational circular dichroism results. Nucleic Acids Res. 1993 Aug 25;21(17):4127–4132. doi: 10.1093/nar/21.17.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Keiderling T. A. Vibrational circular dichroism studies of the A-to-B conformational transition in DNA. Biochemistry. 1992 Oct 27;31(42):10265–10271. doi: 10.1021/bi00157a013. [DOI] [PubMed] [Google Scholar]
- Yang L., Keiderling T. A. Vibrational CD study of the thermal denaturation of poly(rA).poly(rU). Biopolymers. 1993 Feb;33(2):315–327. doi: 10.1002/bip.360330213. [DOI] [PubMed] [Google Scholar]
- Zhong W. X., Gulotta M., Goss D. J., Diem M. DNA solution conformation via infrared circular dichroism: experimental and theoretical results for B-family polymers. Biochemistry. 1990 Aug 14;29(32):7485–7491. doi: 10.1021/bi00484a018. [DOI] [PubMed] [Google Scholar]


