Abstract
Clustering of cell surface adhesion receptors is an essential step in the development of focal contacts, specialized cell-substrate attachment sites where receptors are simultaneously linked to extracellular ligand and cytoskeletal proteins. Previously, we examined the effect of receptor clustering on attachment strength. Here, we employ the numerical methodology developed by Dembo and colleagues (Dembo, M., D.C. Torney, K. Saxman, and D. Hammer. 1988. Proc. R. Soc. Lond. B. 234:55-83) to investigate the kinetics of cell detachment when receptors are clustered into discrete patches. We show that the membrane peeling velocity decreases if receptors are clustered within a patch located inside the contact region. Peeling of clusters is influenced by the chemistry and mechanics of receptor-ligand bonds within the patch. Detachment is also prohibited if the applied tension equals the critical tension of the patch, unless the patch length is small compared with the boundary length over which membrane bending occurs, in which case the patch will peel. Peeling of these short patches only occurs when the mechanical stiffness of clustered bonds is within an optimal range. We compare our model predictions with experimental measurements of T lymphocyte detachment from ICAM-1 substrates. We demonstrate that if discrete patches of receptors are present, detachment occurs through intervals of slow and fast peeling, similar to the dynamics of T lymphocyte peeling, indicating that clustering of LFA-1 receptors is one possible explanation for the observed detachment kinetics in this system.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Nagata K., Yamada K. M. Cell surface receptors for extracellular matrix components. Biochim Biophys Acta. 1990 Feb 28;1031(1):91–110. doi: 10.1016/0304-4157(90)90004-v. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Dembo M., Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984 Jun;45(6):1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Robinson G. S., Bucher N. L., Farmer S. R. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2161–2165. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn P., Kupfer A., Singer S. J. Dynamic membrane-cytoskeletal interactions: specific association of integrin and talin arises in vivo after phorbol ester treatment of peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):497–501. doi: 10.1073/pnas.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A. The behaviour of fibroblasts migrating from chick heart explants: changes in adhesion, locomotion and growth, and in the distribution of actomyosin and fibronectin. J Cell Sci. 1979 Oct;39:149–165. doi: 10.1242/jcs.39.1.149. [DOI] [PubMed] [Google Scholar]
- Cozens-Roberts C., Quinn J. A., Lauffenberger D. A. Receptor-mediated adhesion phenomena. Model studies with the Radical-Flow Detachment Assay. Biophys J. 1990 Jul;58(1):107–125. doi: 10.1016/S0006-3495(90)82357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M., Torney D. C., Saxman K., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests. Biophys J. 1983 Jul;43(1):27–30. doi: 10.1016/S0006-3495(83)84319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Detailed mechanics of membrane-membrane adhesion and separation. I. Continuum of molecular cross-bridges. Biophys J. 1985 Jul;48(1):175–183. doi: 10.1016/S0006-3495(85)83770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Detailed mechanics of membrane-membrane adhesion and separation. II. Discrete kinetically trapped molecular cross-bridges. Biophys J. 1985 Jul;48(1):185–192. doi: 10.1016/S0006-3495(85)83771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath K. R., Edgell C. J., Burridge K. The distribution of distinct integrins in focal contacts is determined by the substratum composition. J Cell Sci. 1989 Jan;92(Pt 1):67–75. doi: 10.1242/jcs.92.1.67. [DOI] [PubMed] [Google Scholar]
- Hammer D. A., Apte S. M. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J. 1992 Jul;63(1):35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990 May;87(9):3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989 Jul;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976 Jun;21(1):129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Lotz M. M., Burdsal C. A., Erickson H. P., McClay D. R. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989 Oct;109(4 Pt 1):1795–1805. doi: 10.1083/jcb.109.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio E. E., Guan J. L., Trevithick J. E., Hynes R. O. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990 Jul;1(8):597–604. doi: 10.1091/mbc.1.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massia S. P., Hubbell J. A. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J Cell Biol. 1991 Sep;114(5):1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massia S. P., Hubbell J. A. Covalent surface immobilization of Arg-Gly-Asp- and Tyr-Ile-Gly-Ser-Arg-containing peptides to obtain well-defined cell-adhesive substrates. Anal Biochem. 1990 Jun;187(2):292–301. doi: 10.1016/0003-2697(90)90459-m. [DOI] [PubMed] [Google Scholar]
- Mooney D., Hansen L., Vacanti J., Langer R., Farmer S., Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992 Jun;151(3):497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- Pecht I., Lancet D. Kinetics of antibody-hapten interactions. Mol Biol Biochem Biophys. 1977;24:306–338. doi: 10.1007/978-3-642-81117-3_9. [DOI] [PubMed] [Google Scholar]
- Rees D. A., Lloyd C. W., Thom D. Control of grip and stick in cell adhesion through lateral relationships of membrane glycoproteins. Nature. 1977 May 12;267(5607):124–128. doi: 10.1038/267124a0. [DOI] [PubMed] [Google Scholar]
- Reszka A. A., Hayashi Y., Horwitz A. F. Identification of amino acid sequences in the integrin beta 1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992 Jun;117(6):1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Streeter H. B., Rees D. A. Fibroblast adhesion to RGDS shows novel features compared with fibronectin. J Cell Biol. 1987 Jul;105(1):507–515. doi: 10.1083/jcb.105.1.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung K. L., Kuhlman P., Maldonado F., Lollo B. A., Chien S., Brian A. A. Force contribution of the LFA-1/ICAM-1 complex to T cell adhesion. J Cell Sci. 1992 Sep;103(Pt 1):259–266. doi: 10.1242/jcs.103.1.259. [DOI] [PubMed] [Google Scholar]
- Tawil N., Wilson P., Carbonetto S. Integrins in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs. focal contacts. J Cell Biol. 1993 Jan;120(1):261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozeren A. Adhesion induced by mobile cross-bridges: steady state peeling of conjugated cell pairs. J Theor Biol. 1989 Sep 11;140(1):1–17. doi: 10.1016/s0022-5193(89)80025-6. [DOI] [PubMed] [Google Scholar]
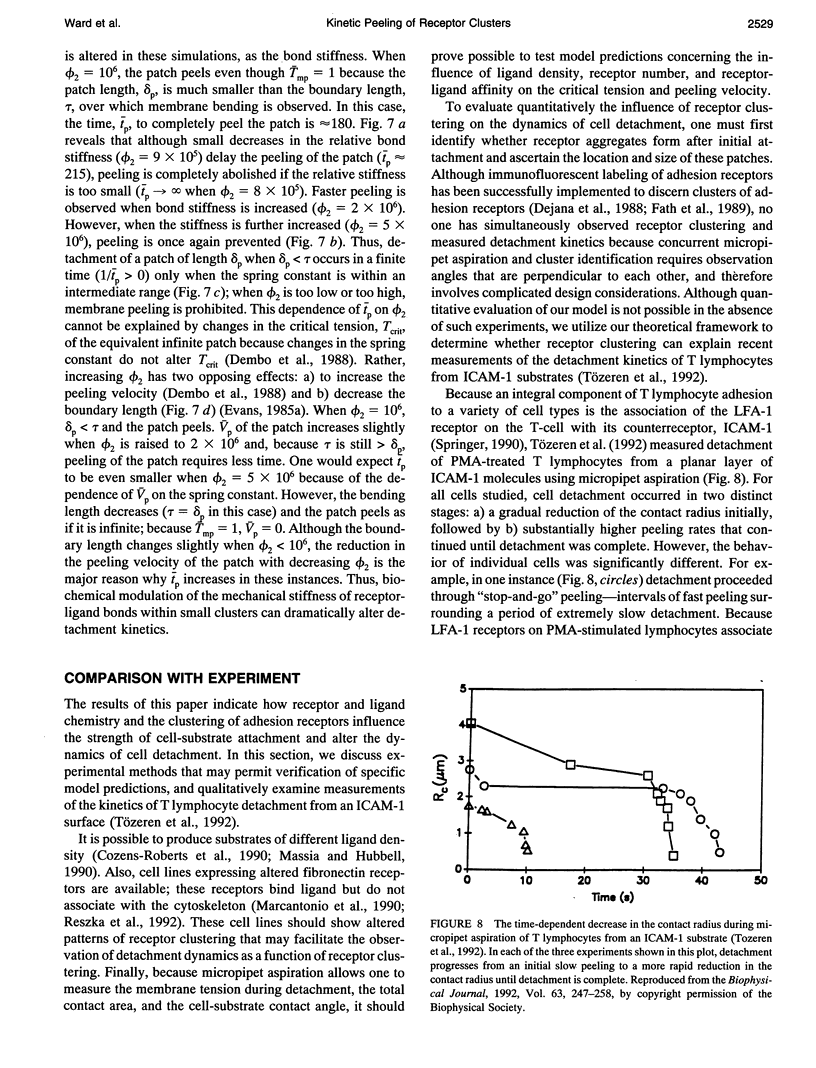
- Tözeren A., Sung K. L., Sung L. A., Dustin M. L., Chan P. Y., Springer T. A., Chien S. Micromanipulation of adhesion of a Jurkat cell to a planar bilayer membrane containing lymphocyte function-associated antigen 3 molecules. J Cell Biol. 1992 Feb;116(4):997–1006. doi: 10.1083/jcb.116.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
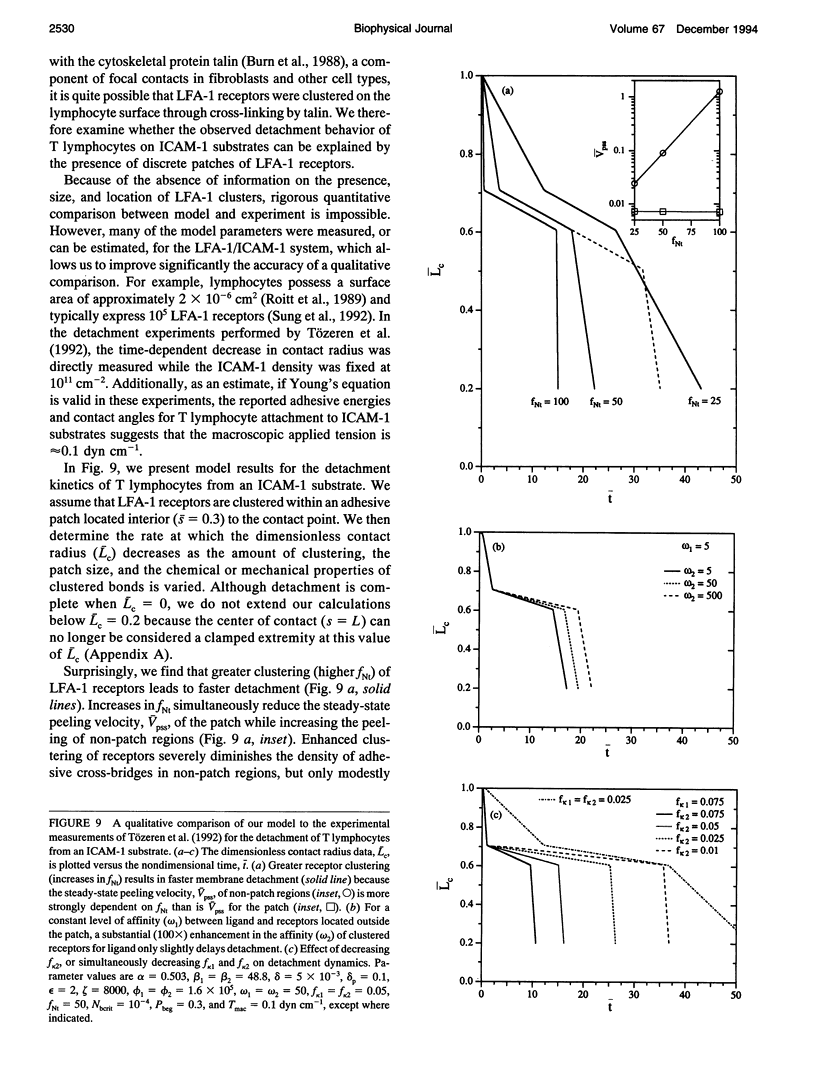
- Ward M. D., Hammer D. A. A theoretical analysis for the effect of focal contact formation on cell-substrate attachment strength. Biophys J. 1993 Mar;64(3):936–959. doi: 10.1016/S0006-3495(93)81456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M., Jordan P. W., O'Neill C. H. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]


