Abstract
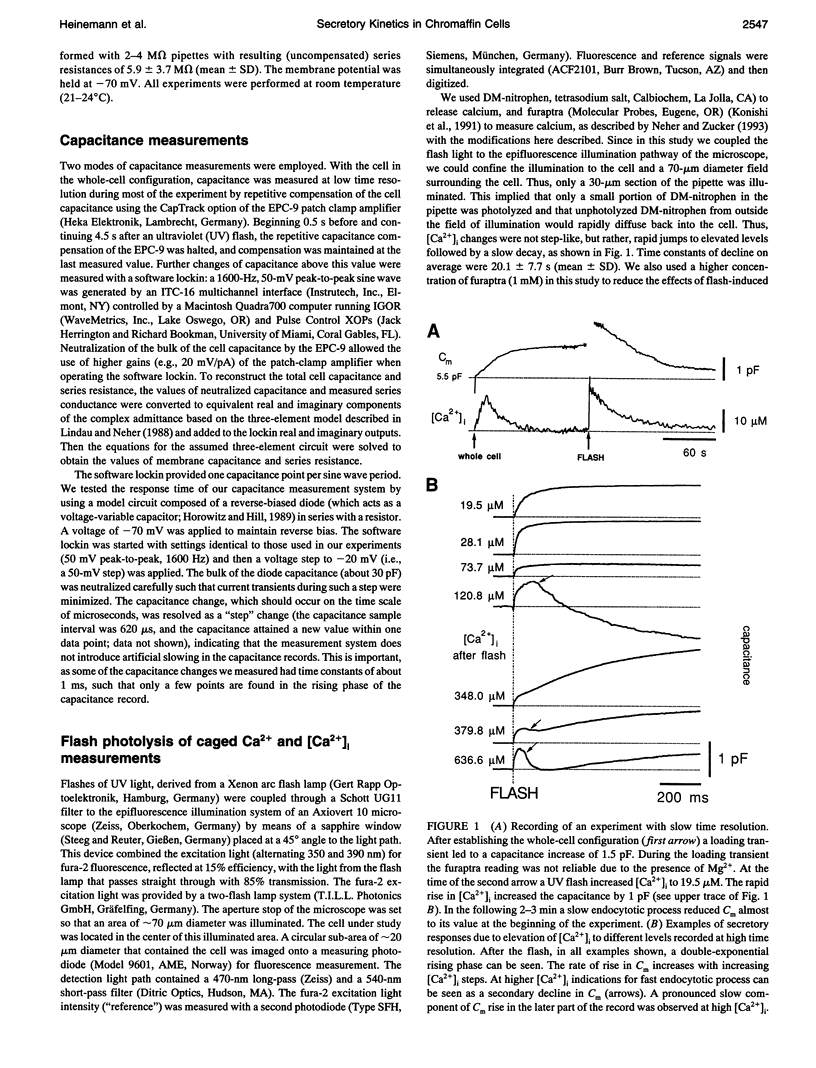
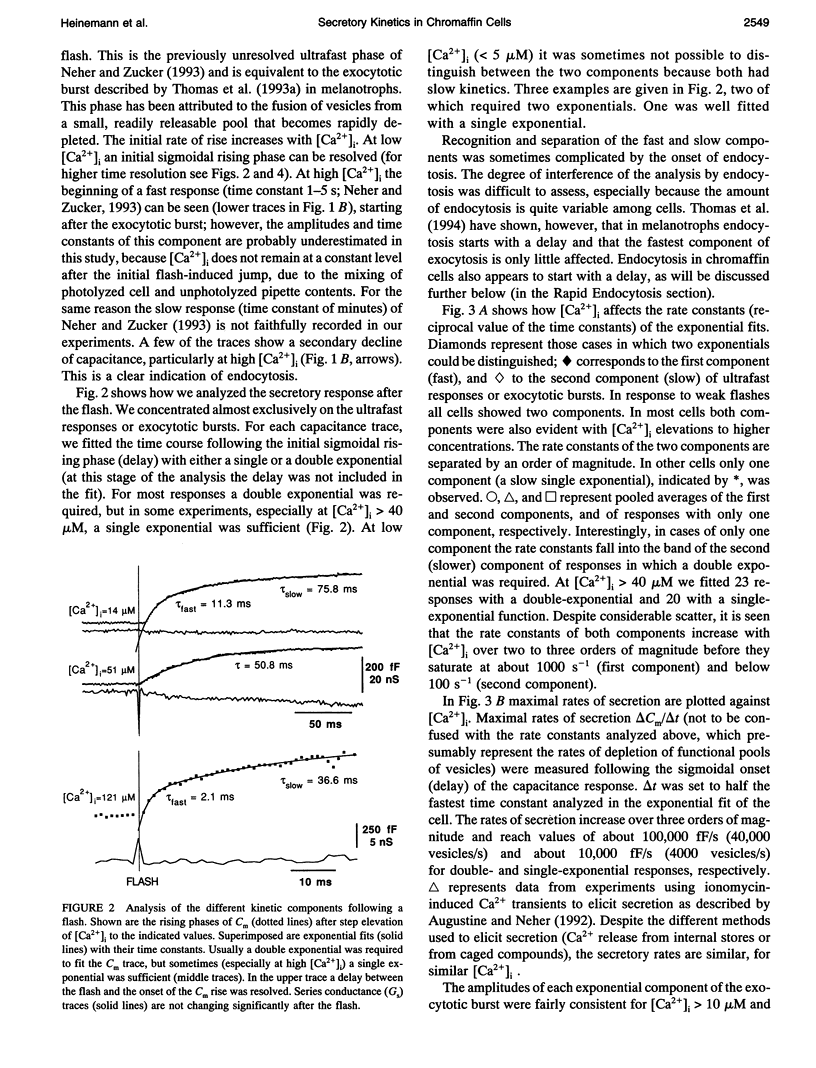
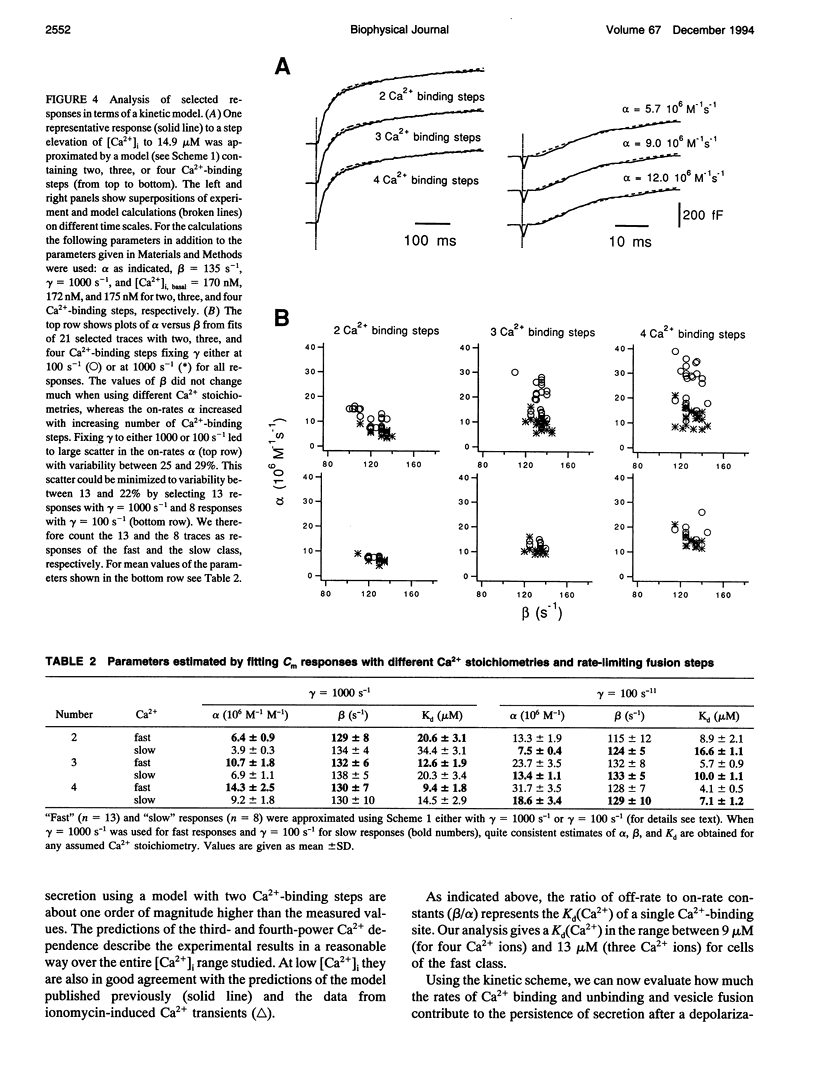
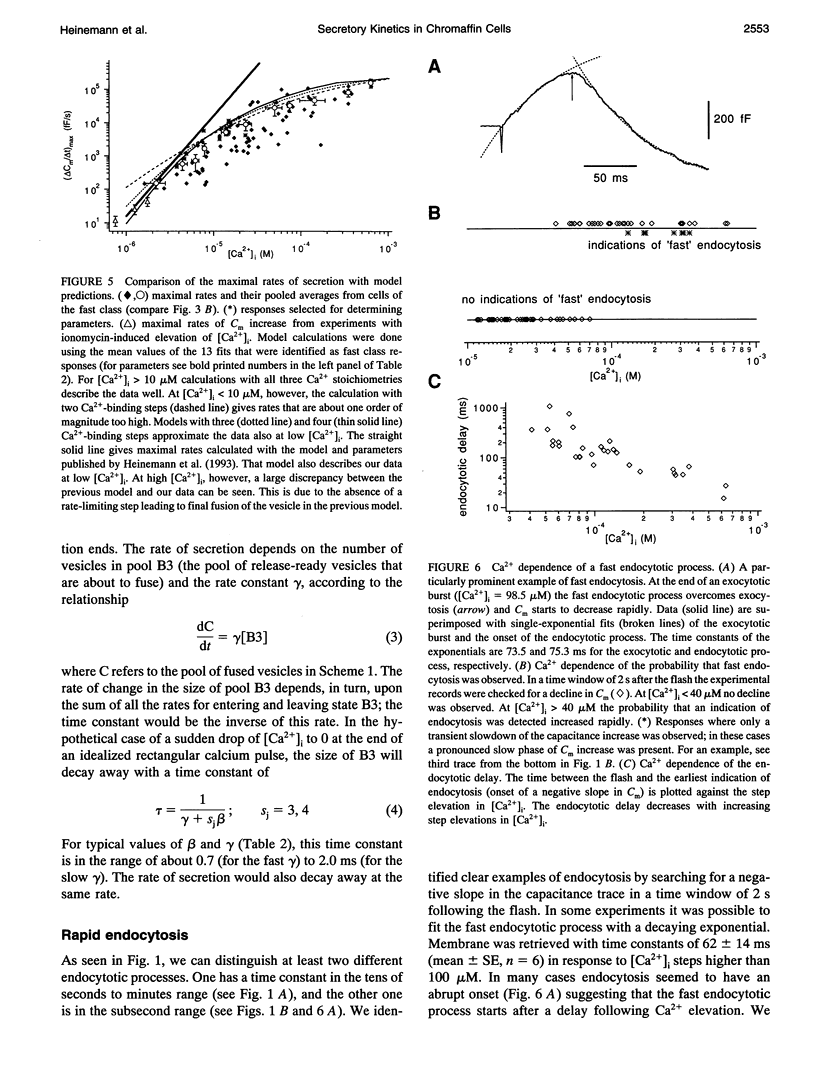
The kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+ were studied by capacitance (Cm) measurements with millisecond time resolution. After elevation of the internal Ca2+ concentration ([Ca2+]i), Cm rises rapidly with one or more exponentials. The time constant of the fastest component decreases for higher [Ca2+]i (range 3-600 microM) over three orders of magnitude before it saturates at approximately 1 ms. The corresponding maximal rates of secretion can be as fast as 100,000 fF/s or 40,000 vesicles/s. There is a Ca(2+)-dependent delay before Cm rises, which may reflect the kinetics of multiple Ca2+ ions binding to the secretory apparatus. The initial rise in Cm is described by models containing a sequence of two to four single Ca(2+)-binding steps followed by a rate-limiting exocytosis step. The predicted Ca2+ dissociation constant (Kd) of a single Ca(2+)-binding site is between 7 and 21 microM. At [Ca2+]i > 30 microM clear indications of a fast endocytotic process complicate the analysis of the secretory response.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol. 1969 Nov;54(5):553–575. doi: 10.1085/jgp.54.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner G. D., Kennedy D. Quantitative aspects of transmitter release. J Cell Biol. 1970 Dec;47(3):585–592. doi: 10.1083/jcb.47.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W. Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J Biol Chem. 1992 Aug 15;267(23):16219–16225. [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Chow R. H., von Rüden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992 Mar 5;356(6364):60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Cohen M. W., Jones O. T., Angelides K. J. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J Neurosci. 1991 Apr;11(4):1032–1039. doi: 10.1523/JNEUROSCI.11-04-01032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov B. A., Südhof T. C. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993 Dec 15;268(35):26386–26390. [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B., Powell M. A. Calpactins: two distinct Ca++-regulated phospholipid- and actin-binding proteins isolated from lung and placenta. J Cell Biol. 1987 Mar;104(3):503–511. doi: 10.1083/jcb.104.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heinemann C., von Rüden L., Chow R. H., Neher E. A two-step model of secretion control in neuroendocrine cells. Pflugers Arch. 1993 Jul;424(2):105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- Jahn R., Südhof T. C. Synaptic vesicles and exocytosis. Annu Rev Neurosci. 1994;17:219–246. doi: 10.1146/annurev.ne.17.030194.001251. [DOI] [PubMed] [Google Scholar]
- Jahromi S. S., Atwood H. L. Three-dimensional ultrastructure of the crayfish neuromuscular apparatus. J Cell Biol. 1974 Nov;63(2 Pt 1):599–613. doi: 10.1083/jcb.63.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe K. T., Zimmerman J. B., Wightman R. M. Principles of voltammetry and microelectrode surface states. J Neurosci Methods. 1993 Jul;48(3):225–240. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- Konishi M., Hollingworth S., Harkins A. B., Baylor S. M. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991 Feb;97(2):271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Zucker R. S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993 Jan;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Pinter M. J. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys J. 1993 Jan;64(1):77–91. doi: 10.1016/S0006-3495(93)81342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H., Hovav G., Parnas I. Effect of Ca2+ diffusion on the time course of neurotransmitter release. Biophys J. 1989 May;55(5):859–874. doi: 10.1016/S0006-3495(89)82885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Thomas P., Lee A. K., Wong J. G., Almers W. A triggered mechanism retrieves membrane in seconds after Ca(2+)-stimulated exocytosis in single pituitary cells. J Cell Biol. 1994 Mar;124(5):667–675. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Almers W. Millisecond studies of secretion in single rat pituitary cells stimulated by flash photolysis of caged Ca2+. EMBO J. 1993 Jan;12(1):303–306. doi: 10.1002/j.1460-2075.1993.tb05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Lee A. K., Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993 Jul;11(1):93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Vitale M. L. Cytoskeleton dynamics during neurotransmitter release. Trends Neurosci. 1993 Nov;16(11):466–472. doi: 10.1016/0166-2236(93)90079-2. [DOI] [PubMed] [Google Scholar]
- Wang W., Creutz C. E. Regulation of the chromaffin granule aggregating activity of annexin I by phosphorylation. Biochemistry. 1992 Oct 20;31(41):9934–9939. doi: 10.1021/bi00156a011. [DOI] [PubMed] [Google Scholar]
- Yamada W. M., Zucker R. S. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992 Mar;61(3):671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Calcium and transmitter release. J Physiol Paris. 1993;87(1):25–36. doi: 10.1016/0928-4257(93)90021-k. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Changes in the statistics of transmitter release during facilitation. J Physiol. 1973 Mar;229(3):787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Effects of photolabile calcium chelators on fluorescent calcium indicators. Cell Calcium. 1992 Jan;13(1):29–40. doi: 10.1016/0143-4160(92)90027-p. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. The calcium concentration clamp: spikes and reversible pulses using the photolabile chelator DM-nitrophen. Cell Calcium. 1993 Feb;14(2):87–100. doi: 10.1016/0143-4160(93)90079-l. [DOI] [PubMed] [Google Scholar]
- von Rüden L., Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993 Nov 12;262(5136):1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]


