Abstract
We reported a case of advanced hepatocellular carcinoma (HCC) in a patient who relapsed after first-line chemoimmunotherapy. Remarkably, the combination of H101 oncolytic virotherapy and tislelizumab successfully induced an abscopal effect. Following this treatment, the patient achieved a 24-month survival period, accompanied by complete regression of distant metastatic lesions. Distinct tumor responses were observed at different sites following H101 injection. Lesions showing regression demonstrated higher infiltration of CD3+ T cells, CD4+ T cells, and eosinophils, along with lower infiltration of neutrophils. Rapid tumor shrinkage was associated with severe local inflammation and a reduction in peripheral white blood cell counts. These findings suggest that oncolytic virotherapy may elicit an abscopal effect by activating and recruiting immune cells into the tumor microenvironment.
Keywords: H101, intra-tumoral injection, abscopal effect, reverse immune resistance, HCC
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, accounting for approximately 75% of all cases [1]. For nearly a decade, the treatment of advanced HCC was limited to sorafenib [2]. Currently, tyrosine kinase inhibitor (TKI)-based systemic therapies such as regorafenib, donafenib, and lenvatinib are widely used; however, resistance remains a major challenge in advanced and unresectable HCC [3]. The combination of TKIs with checkpoint inhibitors (CPIs) has emerged as a new strategy, demonstrating improved overall survival in TKI-resistant cases [4]. Nevertheless, effective treatment options following failure of CPI-based therapies are still lacking.
Oncolytic viruses (OVs) are genetically engineered to selectively infect, replicate in, and lyse tumor cells. To date, nearly 150 clinical trials and over 70 preclinical studies have been conducted to evaluate OVs in various cancers [5]. For HCC specifically, more than 20 clinical trials have been initiated, some showing promising results [6]. H101 (Oncorine), a genetically modified adenovirus with deletions in the E1B and E3 genes, was approved in China in 2005 for the treatment of head, neck, and esophageal cancers [7]. In 2012, H101 was first tested in unresectable HCC. Its combination with transarterial chemoembolization (TACE) significantly improved both overall survival (OS) and progression-free survival (PFS) [8]. A similar trial reported a higher 3-year OS rate with TACE plus H101 (40.5%) compared to TACE alone (22.4%) [9].
In this report, we describe a patient with advanced HCC who experienced disease recurrence following hepatic artery embolization, radical liver resection with cholecystectomy, one cycle of sorafenib, Gamma Knife radiotherapy, and treatment with camrelizumab plus lenvatinib. The patient subsequently achieved partial remission with a combination therapy of intratumoral H101 and anti-PD-1 antibody.
Case report
In July 2022, a 59-year-old male presented with recurrent HCC. His clinical history began in October 2019, when he was diagnosed with a large hepatic mass. CT scans revealed a sizable lesion in the left liver lobe, massive ascites, and elevated prothrombin levels (1050 ng/mL). Emergency hepatic artery embolization was immediately performed. Three days later, he received radiotherapy and began sorafenib (0.4 g, bid) on December 23. By January 2020, follow-up CT showed tumor shrinkage and reduced prothrombin levels (52 ng/mL). On March 2, 2020, he underwent extended liver resection with cholecystectomy. Pathological evaluation confirmed extensive tumor necrosis and residual hepatocellular carcinoma, with negative surgical margins.
In September 2020, chest CT revealed a new ground-glass opacity in the left lower lung. Abdominal MRI detected two new hepatic nodules (up to 1.5 × 2.0 cm), consistent with metastatic disease. Gamma Knife radiotherapy was administered from September 22 to October 30 (330 cGy/F * 10F/11D).
By November 2020, follow-up chest CT showed progression of the pulmonary lesion (1.0 × 1.2 cm), and serum prothrombin increased to 243 ng/mL. The patient was treated with camrelizumab (200 mg IV every 21 days) and lenvatinib (12 mg orally, daily). Disease progression was confirmed in July 2022. MRI revealed: (1) postoperative changes from liver resection and cholecystectomy; (2) multiple intrahepatic metastases; (3) gallbladder duct dilation; (4) splenomegaly; and (5) bilateral renal cysts. He was diagnosed with: (1) intrahepatic and pulmonary metastases post liver cancer surgery (pT3NxM1, stage IV), (2) post-hepatitis B cirrhosis, (3) splenomegaly, and (4) renal cysts. Prothrombin levels rose to 366.7 ng/mL.
Given the failure of prior TKI and CPI therapies, the patient initiated a combination regimen of oncolytic virus and anti-PD-1 immunotherapy (Figure 1). H101 (1.0 mL/injection) was administered intratumorally on July 18, July 19, and August 16, 2022. He concurrently received 11 cycles of tislelizumab (200 mg IV every 21 days) from July 21, 2022 to March 15, 2023. Following the first cycle of systemic immunotherapy, the patient developed grade 3 leukopenia and thrombocytopenia, a high fever (>40°C) lasting seven days, and significant elevations in CRP and calcitonin (Figure 2). He also experienced rapid weight loss (3 kg in 9 days) and persistent hypoalbuminemia. Fever resolved without antibiotic treatment, but fatigue persisted.
Figure 1.
Treatment regime and CT images of injected and noninjected lesion after treatment. A. DCP level during the H101 oncolytic virotherapy and tislelizumab treatment. B. Medical and examination history of the patient. C. MRI images of noninjected and injected lesions after H101 oncolytic virotherapy.
Figure 2.
Serum levels of CRP (A), calcitonin (B) and albumin (C), as well as blood concentrations of WBC (D) of patient. CRP, calcitonin and WBC sharply reached a peak after the administration of H101, then rapidly returned to normal levels in few days. During the treatment, albumin levels remained normal.
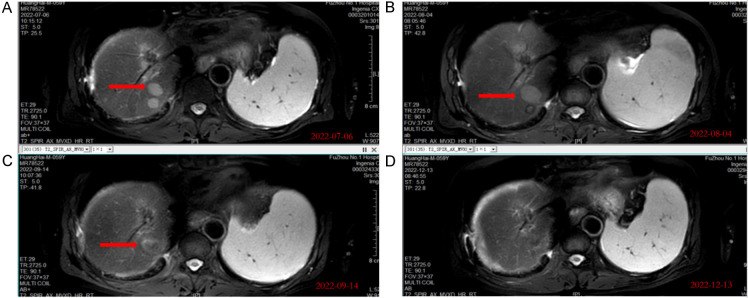
Sixteen days after immunotherapy, imaging showed that the H101-injected tumor had enlarged, though biopsy revealed necrotic HCC cells (Figure 3). Notably, multiple distal metastases showed rapid regression, challenging the traditional view that oncolytic virus therapy induces slow tumor responses. Five months later, MRI confirmed complete disappearance of the segment VII lesion (Figure 4), while the segment VI lesion exhibited slow growth (Figure 5).
Figure 3.
Expression of CD3 and CD4 in relief focus and tolerance focus (×200). A. The expression of CD3 in relief focus. B. The expression of CD3 in tolerance focus. C. The expression of CD4 in relief focus. D. The expression of CD4 in tolerance focus. The relief focus exhibited significantly higher levels of CD4+ and CD3+ T cells compared to the tolerant focus.
Figure 4.
MRI images of section VII liver showed tumor lesion shrinks gradually and vanished after 5 months. A. MRI images of section VII liver in baseline. B. Imaging results obtained after two weeks of the first administration of H101. C. Imaging results obtained after four weeks of the last administration of H101. D. Imaging results obtained after sixteen weeks of the last administration of H101 showed the lesion vanished.
Figure 5.
MRI images of section VI liver. Tumor lesion grows slowly within the 5 months. A. MRI images of section VI liver in baseline. B. Imaging results obtained after two weeks of the first administration of H101. C. Imaging results obtained after four weeks of the last administration of H101. D. Imaging results obtained after sixteen weeks of the last administration of H101.
Pathological analysis of both regressive (“relief”) and non-regressive (“tolerant”) lesions showed intact DNA mismatch repair (MLH1+, PMS2+, MSH2+, MSH6+), microsatellite stability, low expression of CD8 and CD20, and negative PD-1 status. However, the relief focus had significantly more CD4+ and CD3+ T cells than the tolerant focus (Table 1 and Figure 3). High levels of eosinophil were found in the relief lesions, while the tolerant lesions exhibited a lack of eosinophil despite the presence of neutrophil (Table 1).
Table 1.
Pathological features in the two sites
| Relief focus | Tolerance focus | |
|---|---|---|
| CD3 | High | Low |
| CD4 | High | Low |
| Eosinophil | + | - |
| Neutrophil | - | + |
Follow-up scans showed complete regression (CR) of uninjected lesions and partial response (PR) or stable disease (SD) in others, indicating an abscopal effect. Five liver lesions showed CR/PR, and two lung lesions achieved SD. In contrast, the injected tumor continued to grow (PD). The second cycle of immunotherapy was associated with a more intense inflammatory response (marked CRP and WBC elevation), which resolved without intervention. The tumor remained stable at the time of this report (Figure 1).
Discussion
TKI- and CPI-based regimens are currently the most widely used treatments for improving survival in patients with drug-resistant or unresectable HCC. However, recurrence within five years remains common, and there are limited effective therapeutic options for patients who relapse after immunotherapy.
This study, we reported a case of advanced HCC in which the patient, after progressing on both targeted therapy and CPI, achieved an abscopal response and reversal of anti-PD-1 resistance following treatment with intratumoral H101 combined with tislelizumab. Given the limited options for post-immunotherapy progression, the H101 plus tislelizumab regimen was selected. Notably, intratumoral administration of H101 appeared to restore the patient’s antitumor immune function. To our knowledge, this is the first case report documenting the reversal of immune resistance in recurrent, unresectable HCC via oncolytic virotherapy.
Two pathological features are particularly noteworthy in this case. First, H101 administration was followed by a pronounced hyperinflammatory response, which may have contributed to the reactivation of antitumor immunity. The patient’s body temperature rose to 42°C the day after injection and remained at 38-39°C for the subsequent seven days. As oncolytic virotherapy inherently involves a balance between antiviral and antitumor immunity, it is difficult to determine the exact cause of this inflammatory response. However, given the patient’s prior failure on anti-PD-L1 therapy, it is likely that the observed hyperinflammation was driven predominantly by antiviral rather than antitumor mechanisms. Still, a robust antitumor response followed, as evidenced by rapid regression of metastatic lesions at distant sites. This supports the “two-signal” model of immune activation by oncolytic viruses: H101-mediated tumor lysis provides tumor-associated antigens (signal one), while antiviral responses activate costimulatory signals through antigen-presenting cells (signal two). Notably, CRP and procalcitonin levels were even higher following the second H101 injection, suggesting that the initial exposure successfully reprogrammed immune responsiveness. Compared to CPIs and targeted therapies, H101 may offer broader immune activation beyond checkpoint modulation alone.
Second, the combination of H101 and tislelizumab elicited a clear abscopal effect. Distal lesions regressed within 30 days and completely disappeared within six months. Abscopal effects have been previously observed in OV therapies, with or without adjunct cytokines [10,11]. Interestingly, the injected tumor in our case did not respond. H101-induced necrosis may upregulate COX-2 and promote neutrophil infiltration, which in turn could suppress immune cell recruitment via CXCL10 downregulation and neutrophil extracellular traps [12-14]. Future strategies may involve combining H101 with COX inhibitors to enhance CXCL9/CXCL10 expression and improve immune infiltration at the injection site [15].
Despite local progression, H101 may have induced systemic immunity through tumor cell lysis and antigen release, establishing both humoral and cellular antitumor responses. Immunohistochemistry of tumor tissues showed increased infiltration of CD3+ and CD4+ T cells in the regressing (“relief”) lesion compared to the nonresponsive (“tolerant”) lesion. Previous studies also reported that H101 can induce proliferation of CXCR6+ and GZMK+ CD8+ T cells [16]. Thus, T cell recruitment and infiltration may serve as predictive biomarkers for the efficacy of H101-based therapies.
An interesting finding in this case was the higher eosinophil presence in the relief lesion. TNF-α and IFN-γ can activate eosinophils, enhancing CD4+ and CD8+ T cell infiltration and promoting antitumor immunity - consistent with our observations [17]. The disease remains stable at the time of this report, suggesting a sustained “tail effect” from immunotherapy initiated by H101.
In conclusion, H101 restored antitumor immune activity and led to rapid tumor regression in this patient. Oncolytic virotherapy may activate both humoral and cellular immunity and induce an abscopal effect. The combination of H101 and CPI holds promise for patients with immunotherapy-resistant HCC. Further prospective studies are warranted to validate the survival benefits of H101 in this setting.
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Bathon M, Saborowski A. Advances in systemic therapy for the first-line treatment of unresectable HCC. Expert Rev Anticancer Ther. 2021;21:621–628. doi: 10.1080/14737140.2021.1882855. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX, Finn RS. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 5.Chianese A, Santella B, Ambrosino A, Stelitano D, Rinaldi L, Galdiero M, Zannella C, Franci G. Oncolytic viruses in combination therapeutic approaches with epigenetic modulators: past, present, and future perspectives. Cancers (Basel) 2021;13:2761. doi: 10.3390/cancers13112761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L, Lei Y, Huang J, An Y, Ren Y, Chen L, Zhao H, Zheng C. Recent advances in oncolytic virus therapy for hepatocellular carcinoma. Front Oncol. 2023;13:1172292. doi: 10.3389/fonc.2023.1172292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, Robertson AG, Park O, Gulley ML, Heise C, Von Hoff DD, Kaye SB. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 8.Lin XJ, Li QJ, Lao XM, Yang H, Li SP. Transarterial injection of recombinant human type-5 adenovirus H101 in combination with transarterial chemoembolization (TACE) improves overall and progressive-free survival in unresectable hepatocellular carcinoma (HCC) BMC Cancer. 2015;15:707. doi: 10.1186/s12885-015-1715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He CB, Lao XM, Lin XJ. Transarterial chemoembolization combined with recombinant human adenovirus type 5 H101 prolongs overall survival of patients with intermediate to advanced hepatocellular carcinoma: a prognostic nomogram study. Chin J Cancer. 2017;36:59. doi: 10.1186/s40880-017-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havunen R, Santos JM, Sorsa S, Rantapero T, Lumen D, Siurala M, Airaksinen AJ, Cervera-Carrascon V, Tähtinen S, Kanerva A, Hemminki A. Abscopal effect in non-injected tumors achieved with cytokine-armed oncolytic adenovirus. Mol Ther Oncolytics. 2018;11:109–121. doi: 10.1016/j.omto.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakiuchi Y, Kuroda S, Kanaya N, Kumon K, Tsumura T, Hashimoto M, Yagi C, Sugimoto R, Hamada Y, Kikuchi S, Nishizaki M, Kagawa S, Tazawa H, Urata Y, Fujiwara T. Local oncolytic adenovirotherapy produces an abscopal effect via tumor-derived extracellular vesicles. Mol Ther. 2021;29:2920–2930. doi: 10.1016/j.ymthe.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: a review. J Cell Physiol. 2019;234:5683–5699. doi: 10.1002/jcp.27411. [DOI] [PubMed] [Google Scholar]
- 13.Bronger H, Singer J, Windmüller C, Reuning U, Zech D, Delbridge C, Dorn J, Kiechle M, Schmalfeldt B, Schmitt M, Avril S. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br J Cancer. 2016;115:553–63. doi: 10.1038/bjc.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of Neutrophil Extracellular Traps (NETs) in tumor progression and metastasis. Front Immunol. 2020;11:1749. doi: 10.3389/fimmu.2020.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronger H, Kraeft S, Schwarz-Boeger U, Cerny C, Stöckel A, Avril S, Kiechle M, Schmitt M. Modulation of CXCR3 ligand secretion by prostaglandin E2 and cyclooxygenase inhibitors in human breast cancer. Breast Cancer Res. 2012;14:R30. doi: 10.1186/bcr3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Qian L, Chen K, Gu S, Meng Z, Wang J, Li Y, Wang P. Oncolytic adenovirus in treating malignant ascites: a phase II trial and longitudinal single-cell study. Mol Ther. 2024;32:2000–2020. doi: 10.1016/j.ymthe.2024.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grisaru-Tal S, Dulberg S, Beck L, Zhang C, Itan M, Hediyeh-Zadeh S, Caldwell J, Rozenberg P, Dolitzky A, Avlas S, Hazut I, Gordon Y, Shani O, Tsuriel S, Gerlic M, Erez N, Jacquelot N, Belz GT, Rothenberg ME, Davis MJ, Yu H, Geiger T, Madi A, Munitz A. Metastasis-entrained eosinophils enhance lymphocyte-mediated antitumor immunity. Cancer Re. 2021;81:5555–5571. doi: 10.1158/0008-5472.CAN-21-0839. [DOI] [PubMed] [Google Scholar]