Abstract
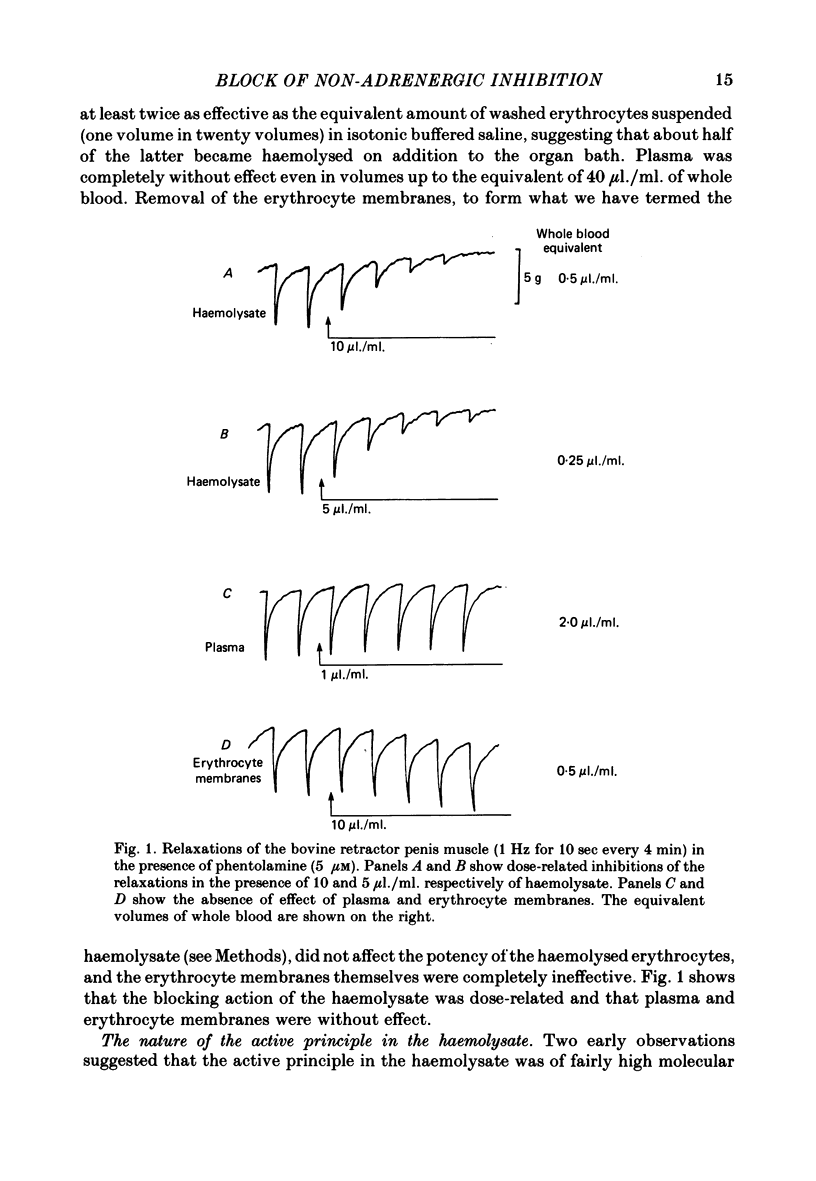
1. A preparation of haemolysed rat erythrocytes (the haemolysate) blocked the relaxations of both the bovine retractor penis and the rat anococcygeus muscles in response to field stimulation of their non-adrenergic inhibitory nerves. The effective concentration range was 5-20 μl./ml. of haemolysate, equivalent to 0·25-1·0 μl./ml. of blood. The active principle in the haemolysate was a non-dialysable, heat-labile material of molecular weight between 50,000 and 100,000 daltons. If, as appeared probable, the active component of the haemolysate was oxyhaemoglobin, its effective blocking concentration was 0·5-2 μM.
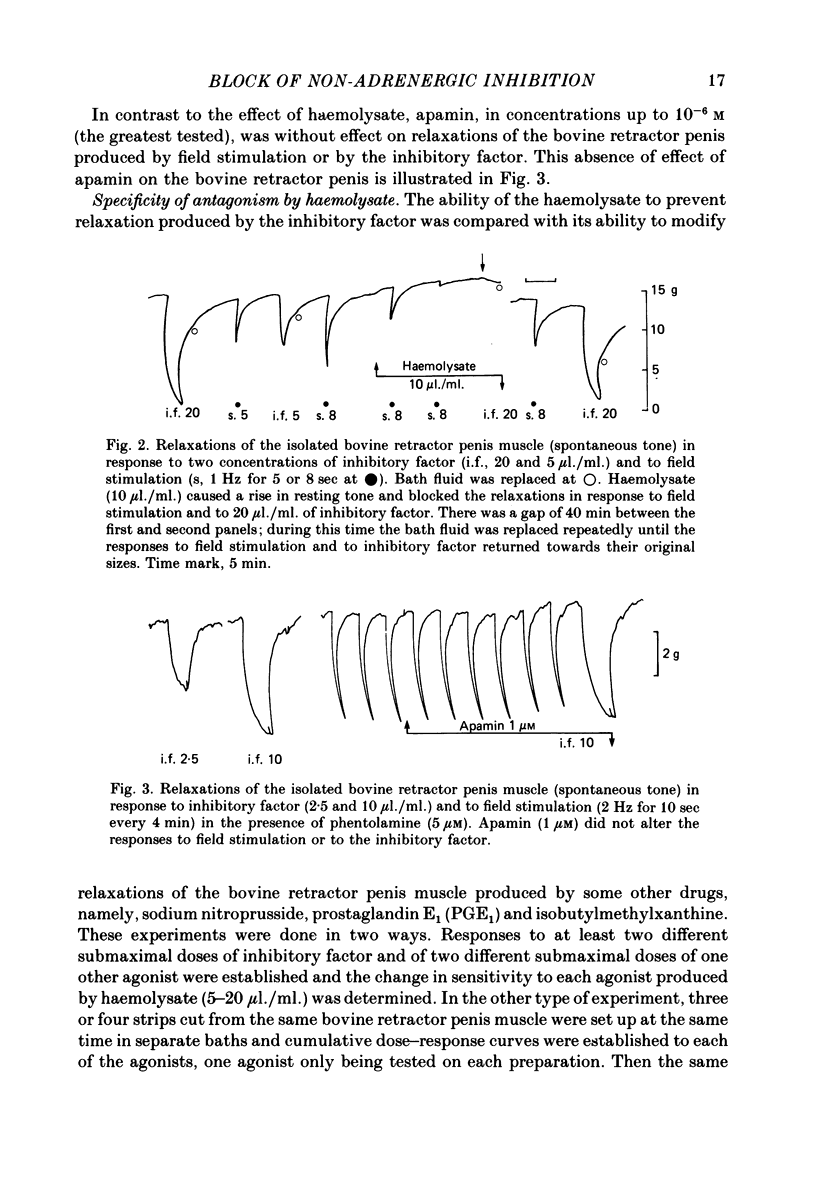
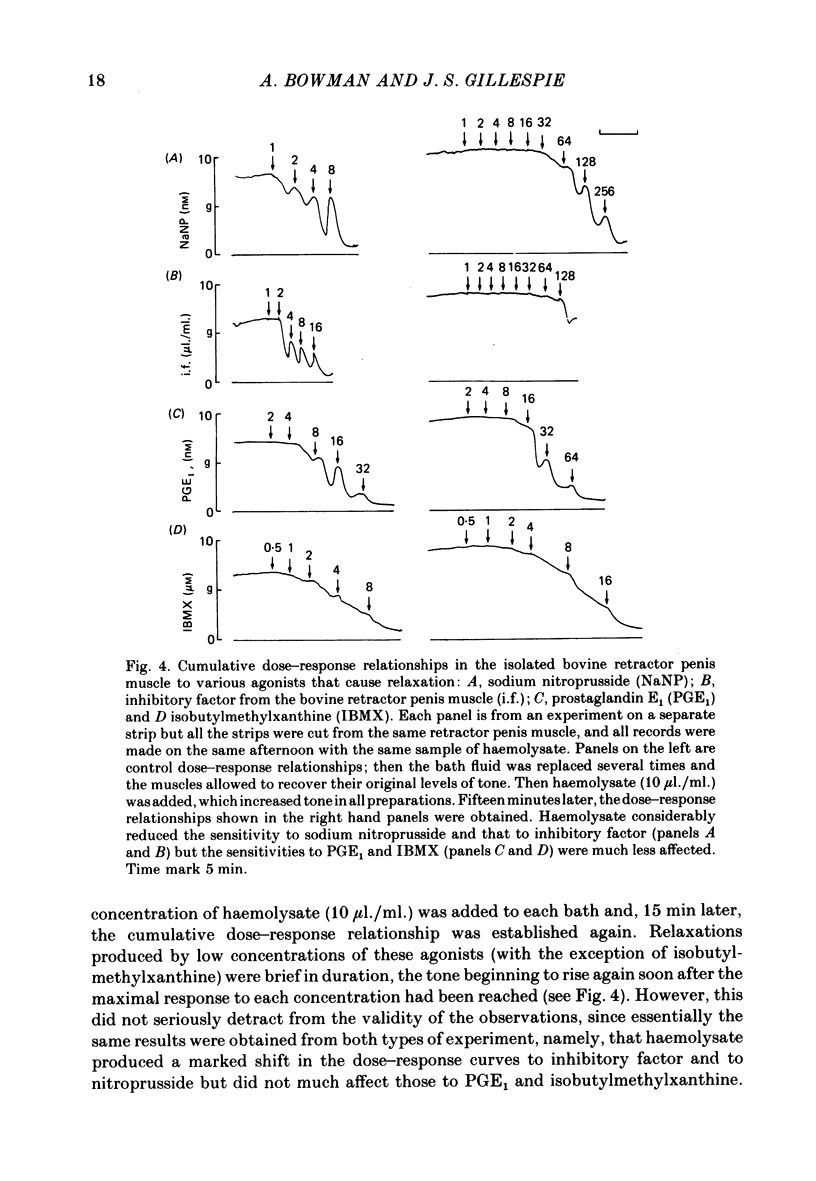
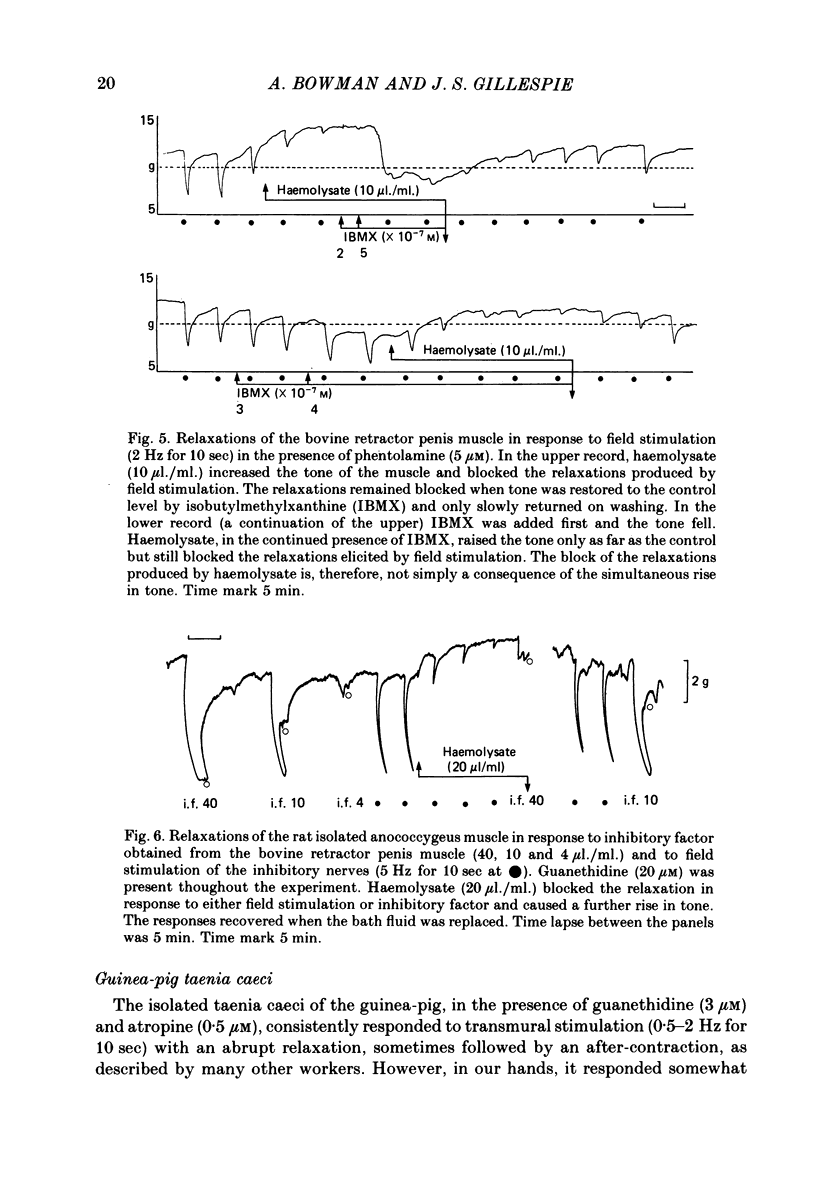
2. Haemolysate (5-20 μl./ml.) also blocked the relaxation of both the bovine retractor penis and the rat anococcygeus to the inhibitory factor extracted from the bovine retractor penis, an observation supporting the possibility that this inhibitory factor may be the transmitter released by the inhibitory nerves in these tissues. In the bovine retractor penis, haemolysate was also effective in blocking relaxations in response to sodium nitroprusside, but relaxations produced by prostaglandin E1 or isobutylmethylxanthine were unchanged or only slightly reduced.
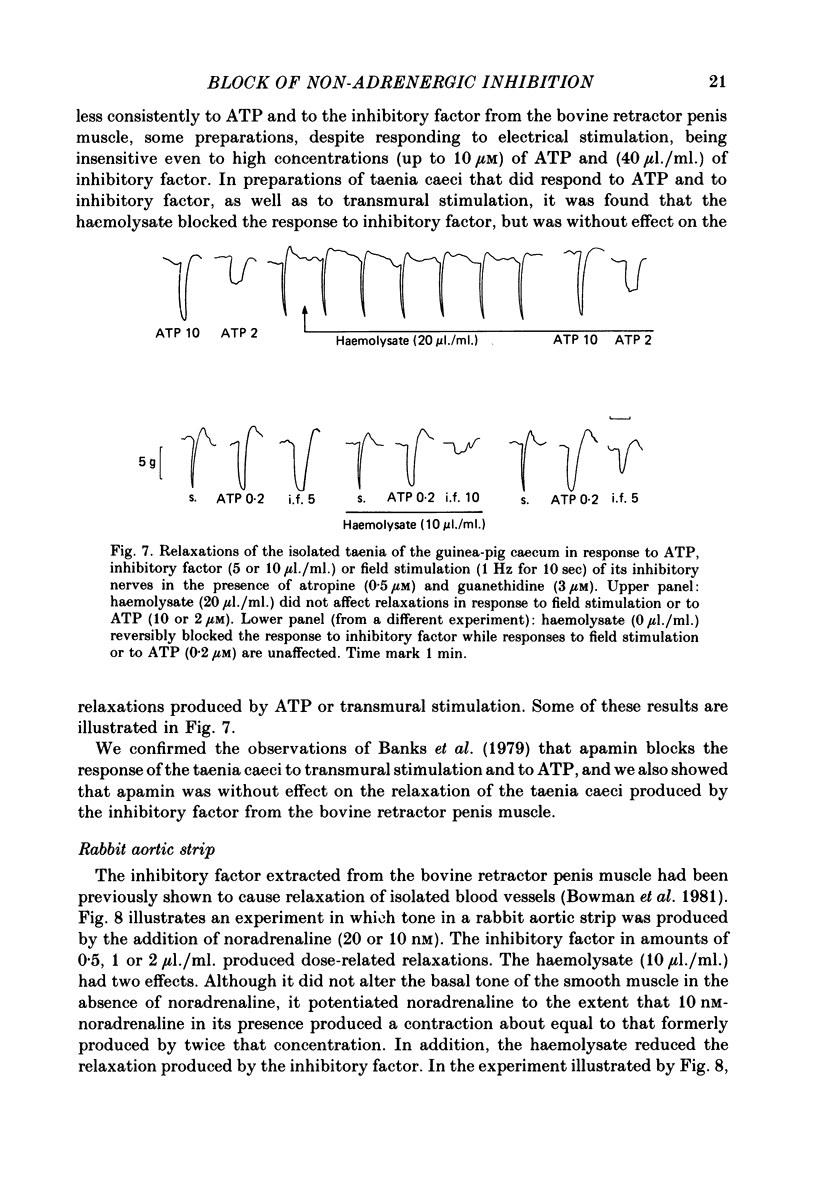
3. In contrast, in the taenia of the guinea-pig caecum, haemolysate did not block the non-adrenergic inhibitory response to field stimulation, nor the relaxation produced by ATP, although it did block the relaxation produced by the inhibitory factor.
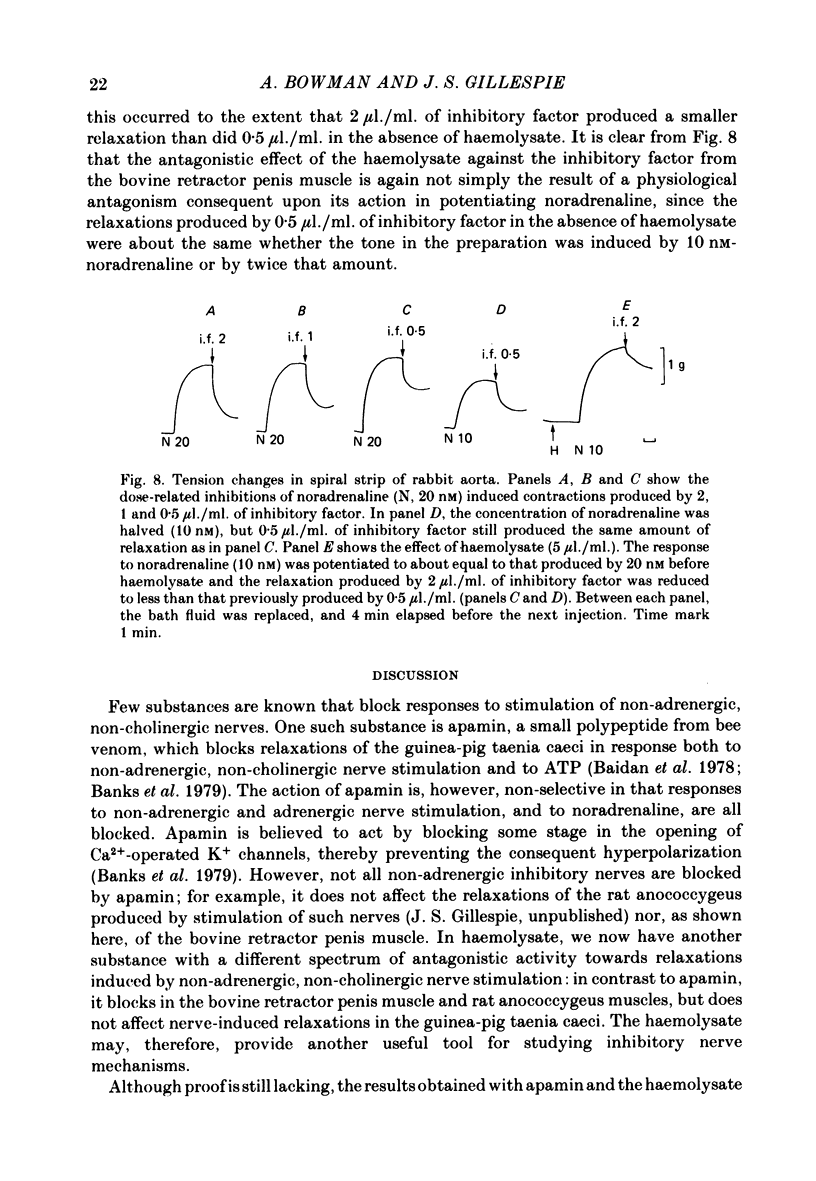
4. In spiral strips of isolated rabbit aorta, haemolysate (10 μl./ml.) increased the contraction produced by noradrenaline and blocked the relaxation produced by the inhibitory factor. These were shown to be independent effects.
5. Apamin, which blocked the relaxation of the taenia of the guinea-pig caecum elicited by either ATP or field stimulation of its non-adrenergic nerves, was without effect on relaxations of the bovine retractor penis or rat anococcygeus muscles in response to field stimulation of inhibitory nerves or to inhibitory factor.
6. These differences in the blocking effects of apamin and haemolysate suggest either that the transmitter in the bovine retractor penis and rat anococcygeus differs from that in the guinea-pig taenia, or, if the transmitter is the same, then its mechanism of action differs.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Killick S. W., Aboo Aar M. Extraction from ox retractor penis of an inhibitory substance which mimics its atropine-resistant neurogenic relaxation. Br J Pharmacol. 1975 Jul;54(3):409–410. doi: 10.1111/j.1476-5381.1975.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baidan L. V., Vladimirova I. A., Miroshnikov A. I., Taran G. A. Deistvie apamina na sinapticheskuiu peredachu v razlichnykh tipakh sinapsov. Dokl Akad Nauk SSSR. 1978 Aug 11;241(5):1224–1227. [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. Actions on the cardiovascular system of an inhibitory material extracted from the bovine retractor penis. Br J Pharmacol. 1981 Feb;72(2):365–372. doi: 10.1111/j.1476-5381.1981.tb09136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. The inhibitory material in extracts from the bovine retractor penis muscle is not an adenine nucleotide. Br J Pharmacol. 1979 Nov;67(3):327–328. doi: 10.1111/j.1476-5381.1979.tb08683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Burnstock G. Evidence in support of the P1/P2 purinoceptor hypothesis in the guinea-pig taenia coli. Br J Pharmacol. 1981 Jul;73(3):617–624. doi: 10.1111/j.1476-5381.1981.tb16796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R. Evidence for purinergic innervation of the anococcygeus muscle. Br J Pharmacol. 1978 Sep;64(1):13–20. doi: 10.1111/j.1476-5381.1978.tb08635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- De Venuto F., Zuck T. F., Zegna A. I., Moores W. Y. Characteristics of stroma-free hemoglobin prepared by crystallization. J Lab Clin Med. 1977 Mar;89(3):509–516. [PubMed] [Google Scholar]
- Echlin F. Experimental vasospasm, acute and chronic, due to blood in the subarachnoid space. J Neurosurg. 1971 Dec;35(6):646–656. doi: 10.3171/jns.1971.35.6.0646. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Hunter J. C., Martin W. Some physical and chemical properties of the smooth muscle inhibitory factor in extracts of the bovine retractor penis muscle. J Physiol. 1981 Jun;315:111–125. doi: 10.1113/jphysiol.1981.sp013736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material extracted from the bovine retractor penis and rat anococcygeus muscles [proceedings]. J Physiol. 1978 Jul;280:45P–46P. [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material from the bovine retractor penis and rat anococcygeus muscles. J Physiol. 1980 Dec;309:55–64. doi: 10.1113/jphysiol.1980.sp013493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge E., Sjöstrand N. O. Contraction and relaxation of the retractor penis muscle and the penile artery of the bull. Acta Physiol Scand Suppl. 1974;420:1–88. [PubMed] [Google Scholar]
- Lee T. J., Hume W. R., Su C., Bevan J. A. Neurogenic vasodilation of cat cerebral arteries. Circ Res. 1978 Apr;42(4):535–542. doi: 10.1161/01.res.42.4.535. [DOI] [PubMed] [Google Scholar]
- Osaka K. Prolonged vasospasm produced by the breakdown products of erythrocytes. J Neurosurg. 1977 Sep;47(3):403–411. doi: 10.3171/jns.1977.47.3.0403. [DOI] [PubMed] [Google Scholar]
- Starling L. M., Boullin D. J., Grahame-Smith D. G., Adams C. B., Gye R. S. Responses of isolated human basilar arteries to 5-hydroxytryptamine, noradrenaline, serum, platelets, and erythrocytes. J Neurol Neurosurg Psychiatry. 1975 Jul;38(7):650–656. doi: 10.1136/jnnp.38.7.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanishima T. Cerebral vasospasm: contractile activity of hemoglobin in isolated canine basilar arteries. J Neurosurg. 1980 Dec;53(6):787–793. doi: 10.3171/jns.1980.53.6.0787. [DOI] [PubMed] [Google Scholar]
- Toda N. Non-adrenergic, non-cholinergic innervation in monkey and human cerebral arteries. Br J Pharmacol. 1981 Feb;72(2):281–283. doi: 10.1111/j.1476-5381.1981.tb09126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellum G. R., Irvine T. W., Jr, Zervas N. T. Dose responses of cerebral arteries of the dog, rabbit, and man to human hemoglobin in vitro. J Neurosurg. 1980 Oct;53(4):486–490. doi: 10.3171/jns.1980.53.4.0486. [DOI] [PubMed] [Google Scholar]


