Abstract
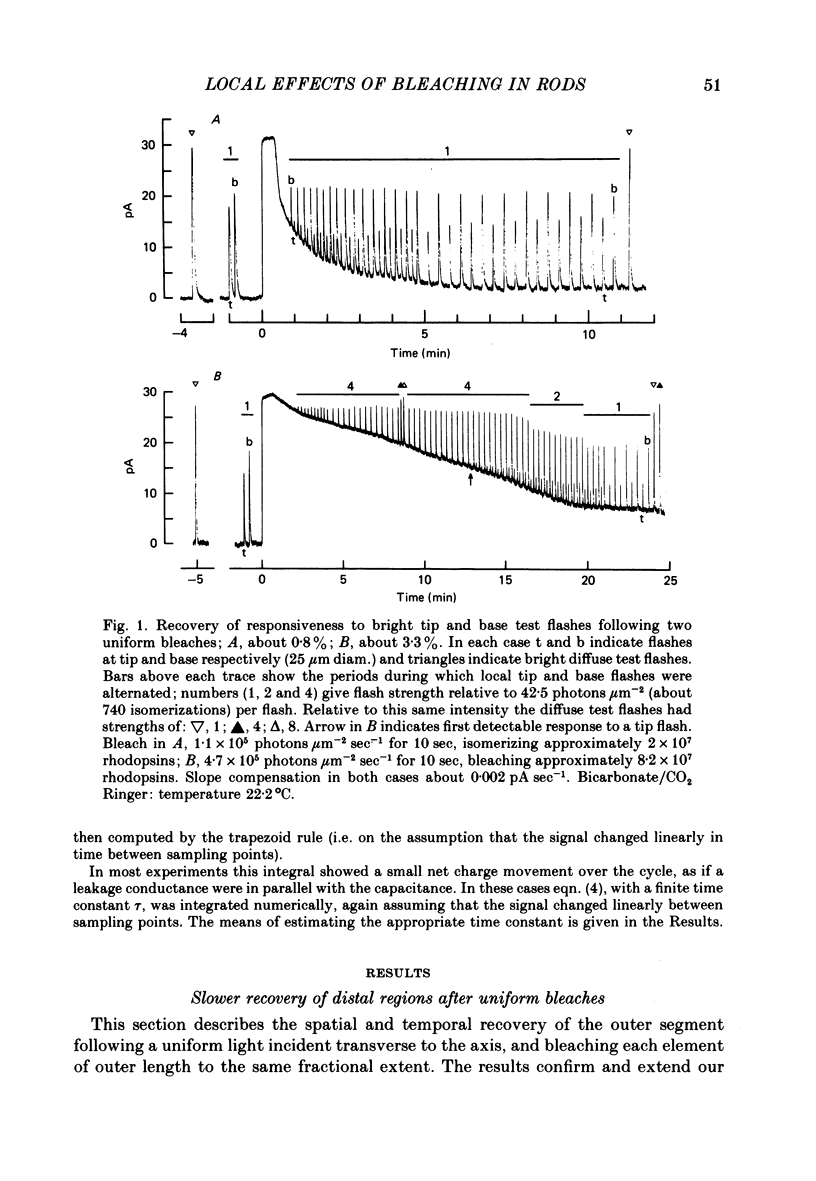
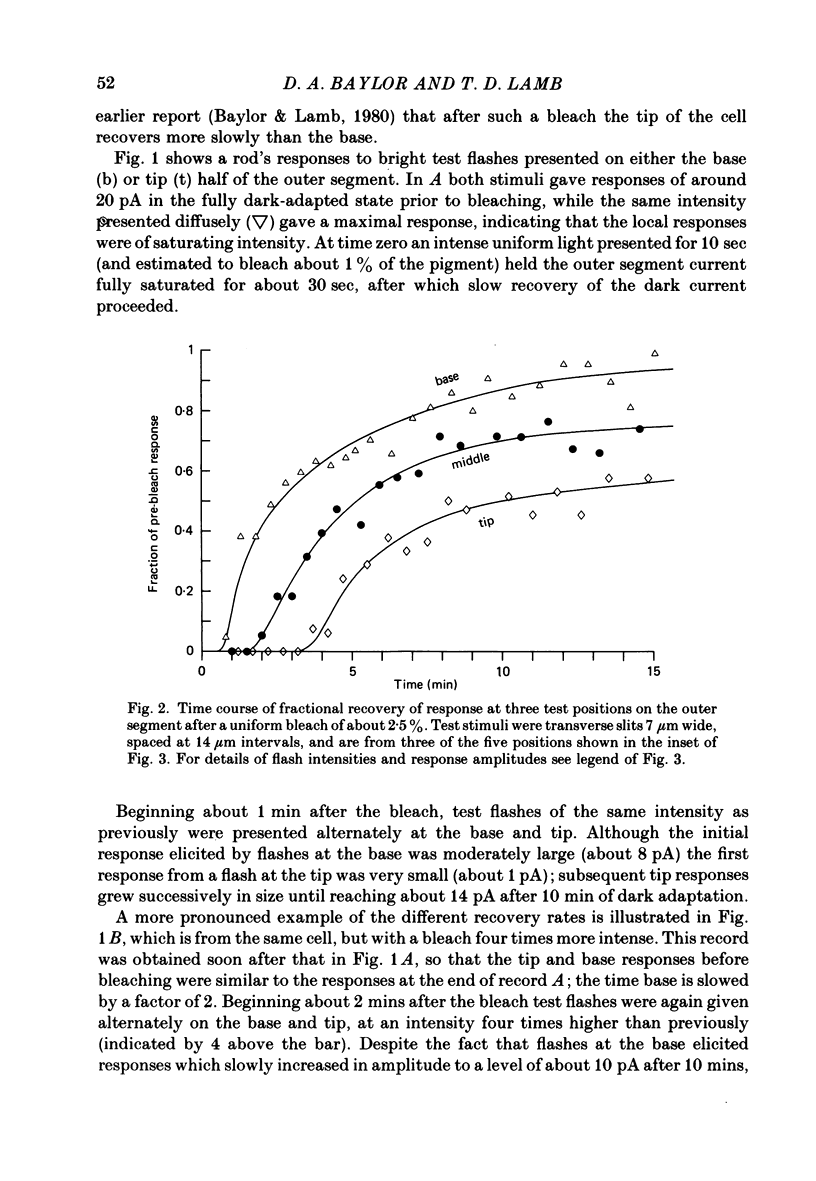
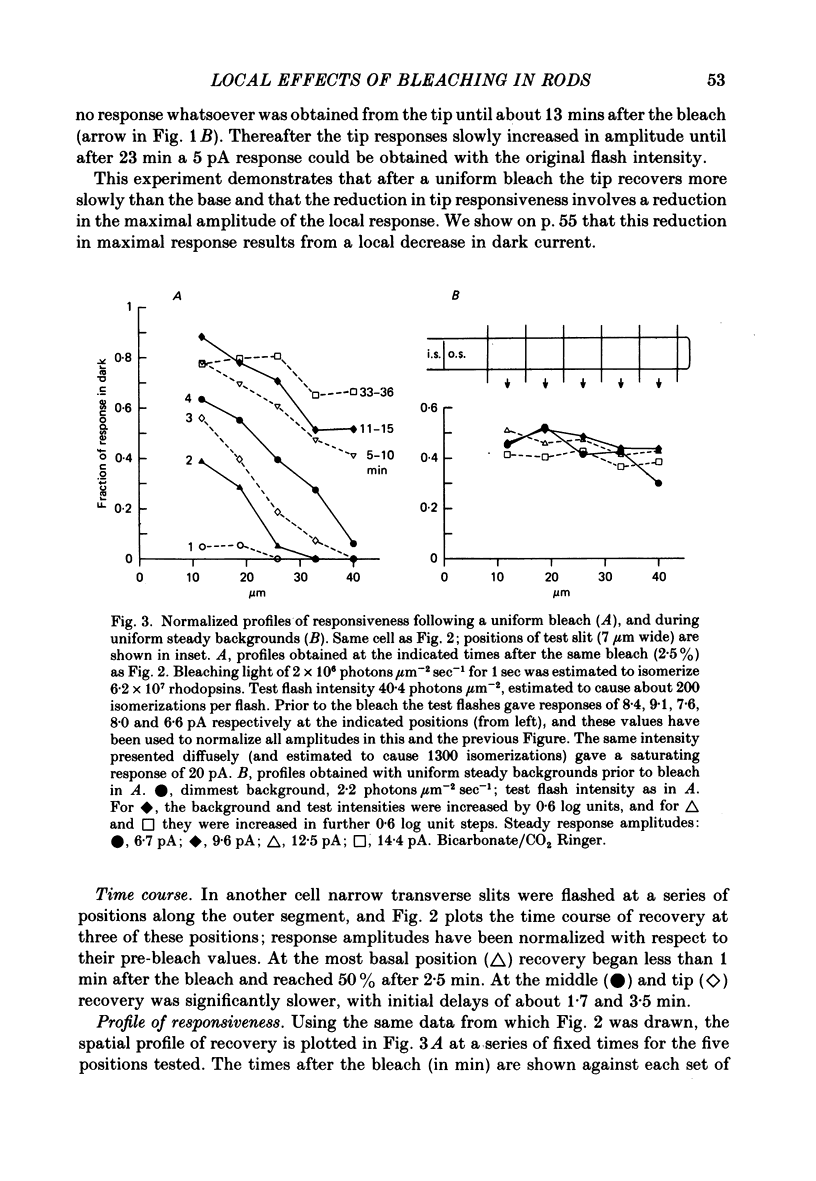
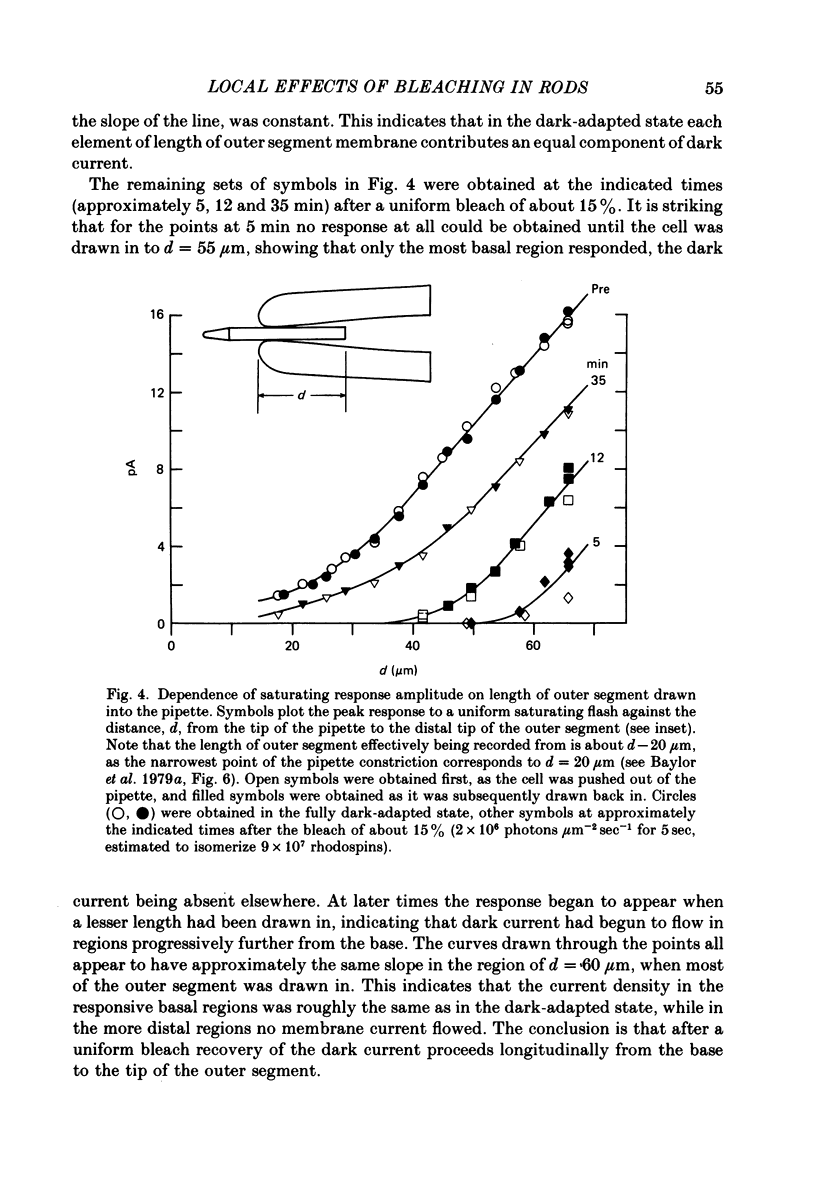
1. Suction electrode recordings were used to study the recovery of responsiveness in single toad rods after bleaching a small fraction (less than 5%) of the rhodopsin. 2. After a spatially uniform bleach that initially abolished the dark current over the entire length of the outer segment, the more proximal regions recovered faster than the more distal regions. For a time the most basal region was almost fully recovered while the tip remained fully saturated. 3. Such a gradient of responsiveness did not occur during uniform steady background illumination of dark-adapted cells. 4. The entire outer segment recovered uniformly after a longitudinally graded bleach that simulated the pattern produced by self-screening in the intact eye. 5. The recovery of the distal end of the outer segment was not affected by a bleach at the proximal end. This suggests that the differences in recovery rate reflect intrinsic local properties of the outer segment rather than longitudinal diffusion of a substance from the inner segment. 6. For at least the first 3 min after bleaching with a narrow transverse slit the reduction of responsiveness remained most pronounced in the bleached region, suggesting that this effect of bleaching does not spread extensively. 7. The increased noise induced by bleaching is shown to originate locally in the bleached region of outer segment. 8. When the tip was locally saturated after a bleach or during steady light, the current recorded from the tip was predominantly capacitive, resulting from intracellular voltage change. This indicates that when the dark current is abolished the outer segment plasma membrane has negligible leakage conductance.
Full text
PDF


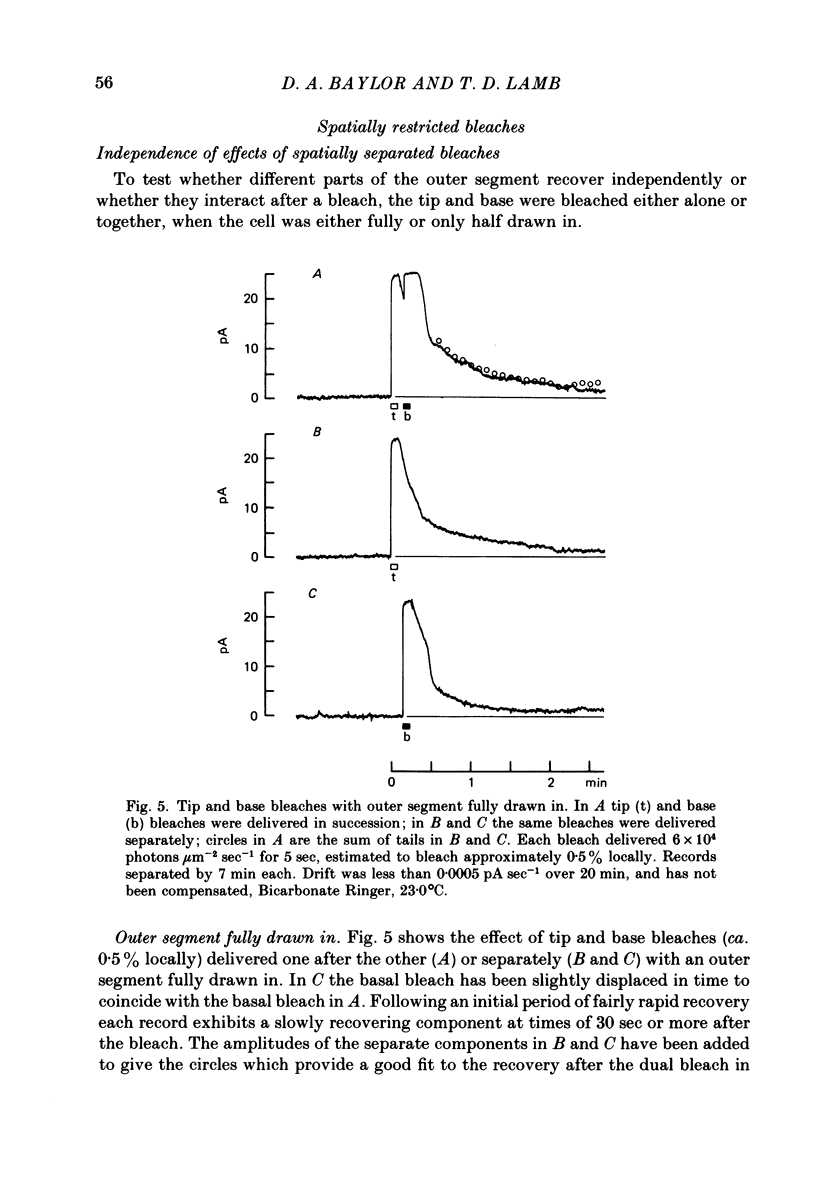
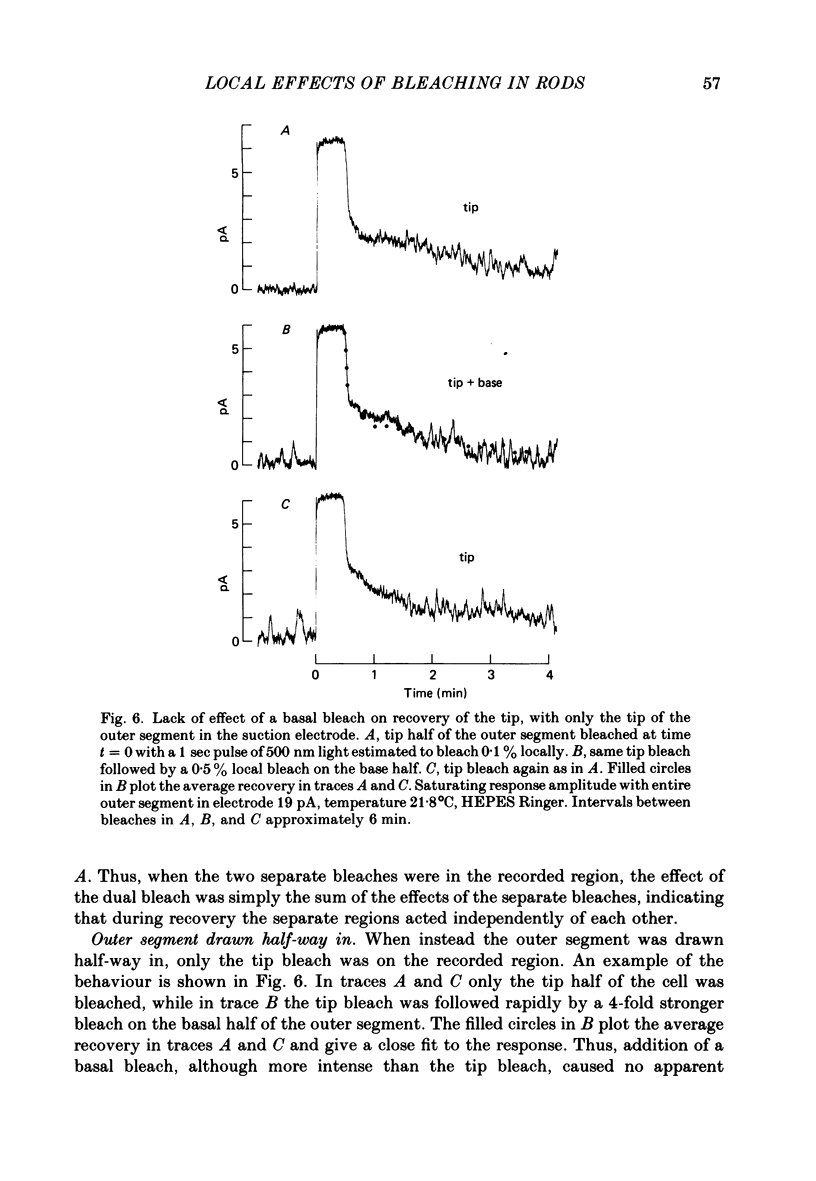
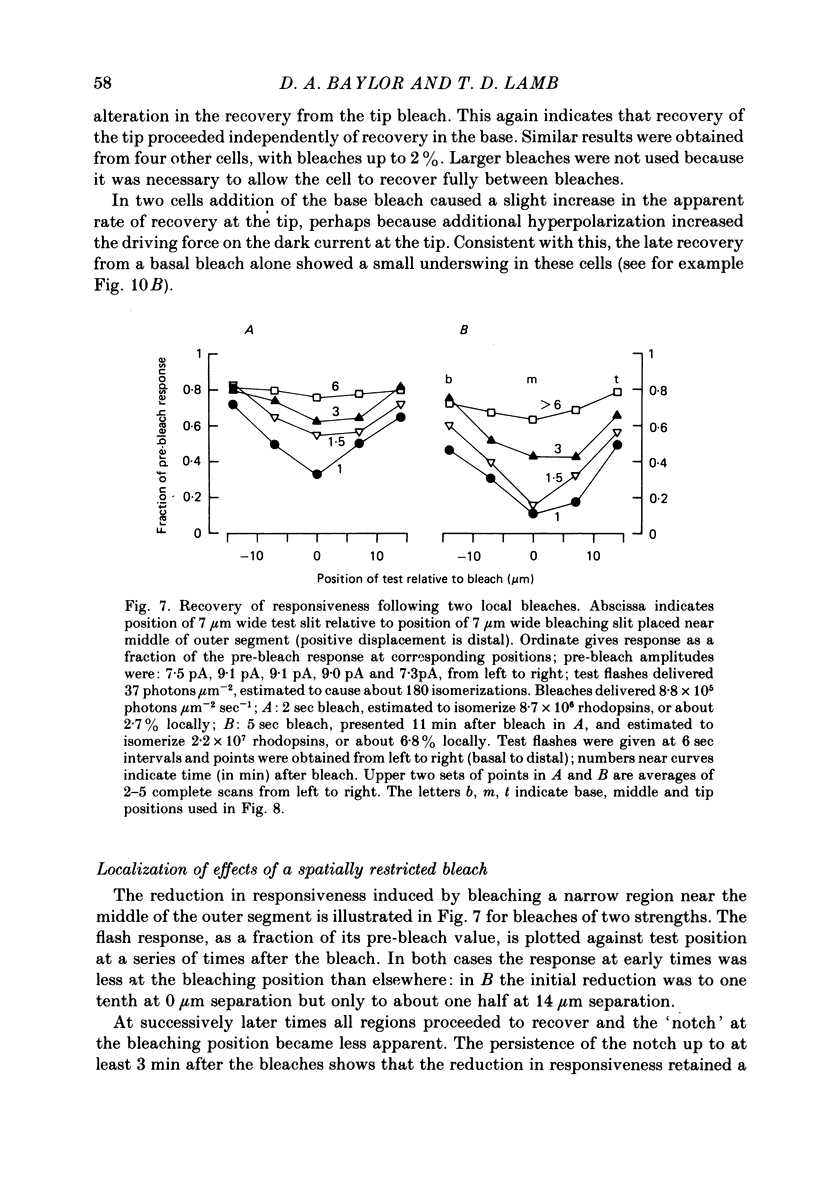
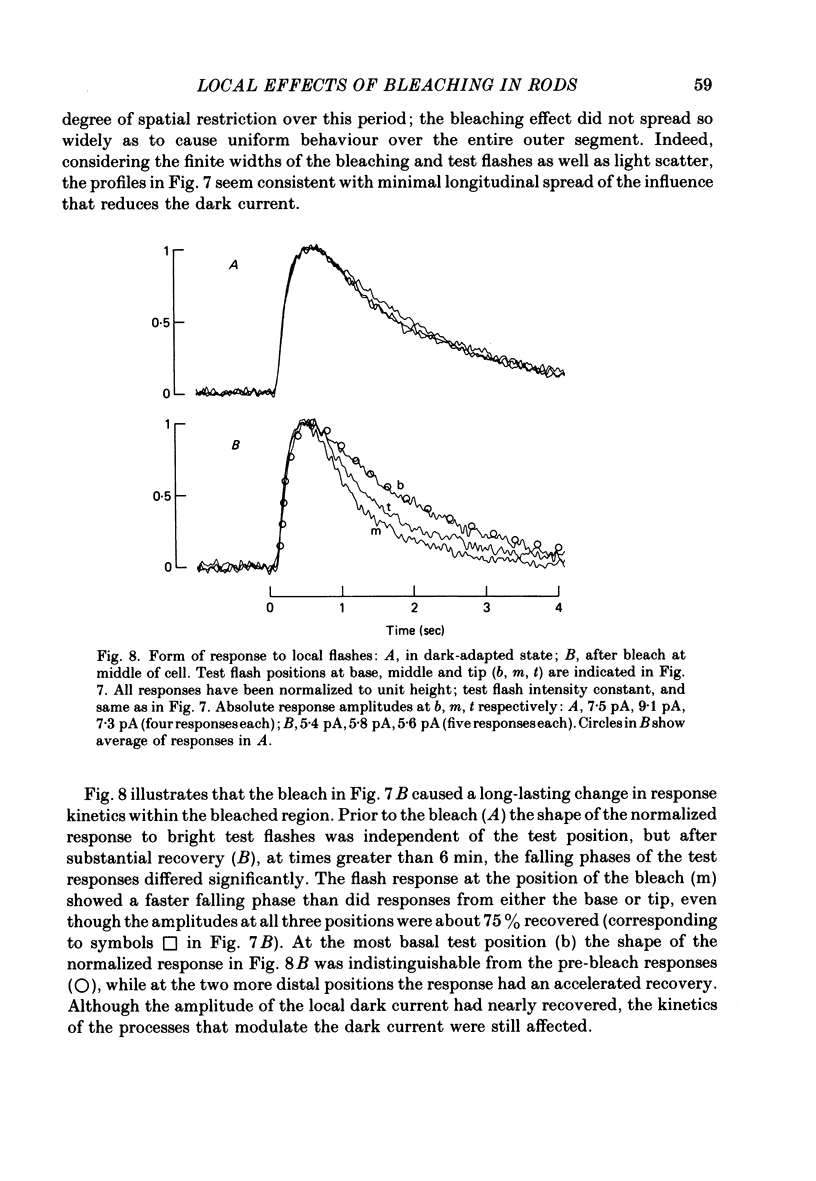
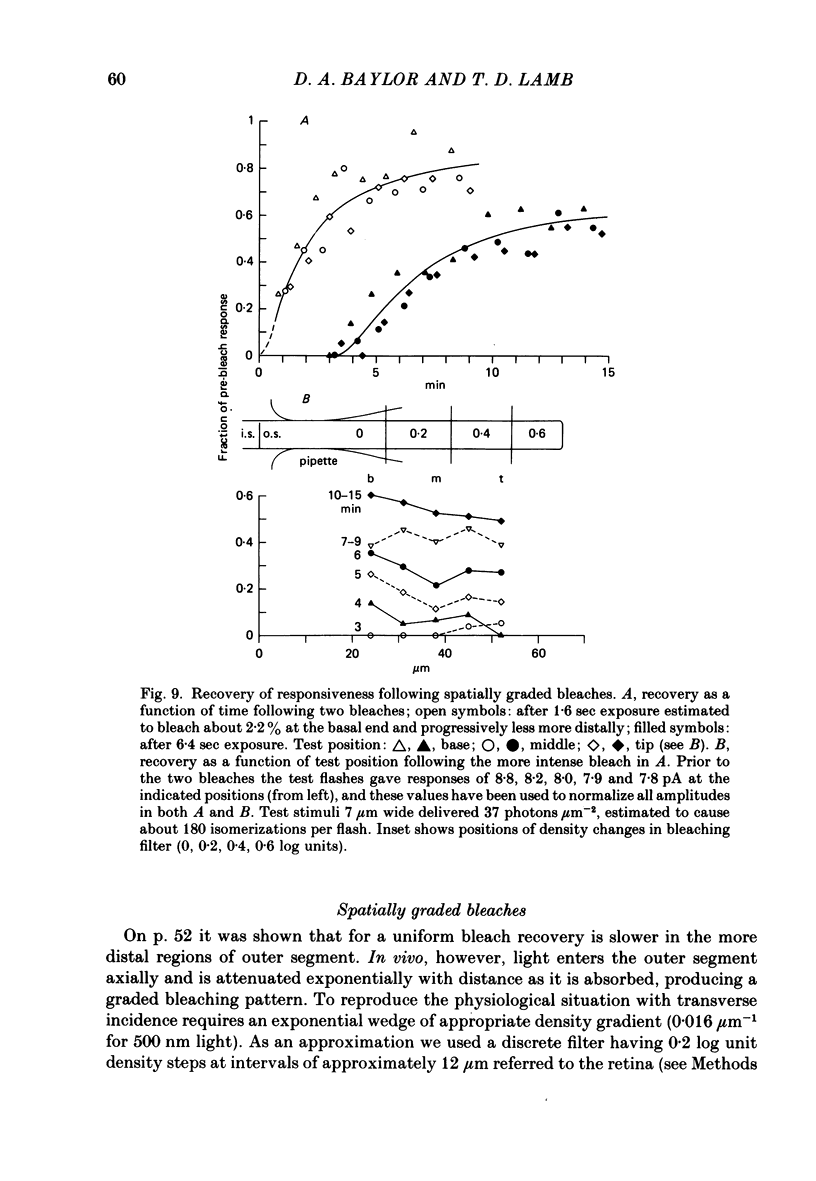

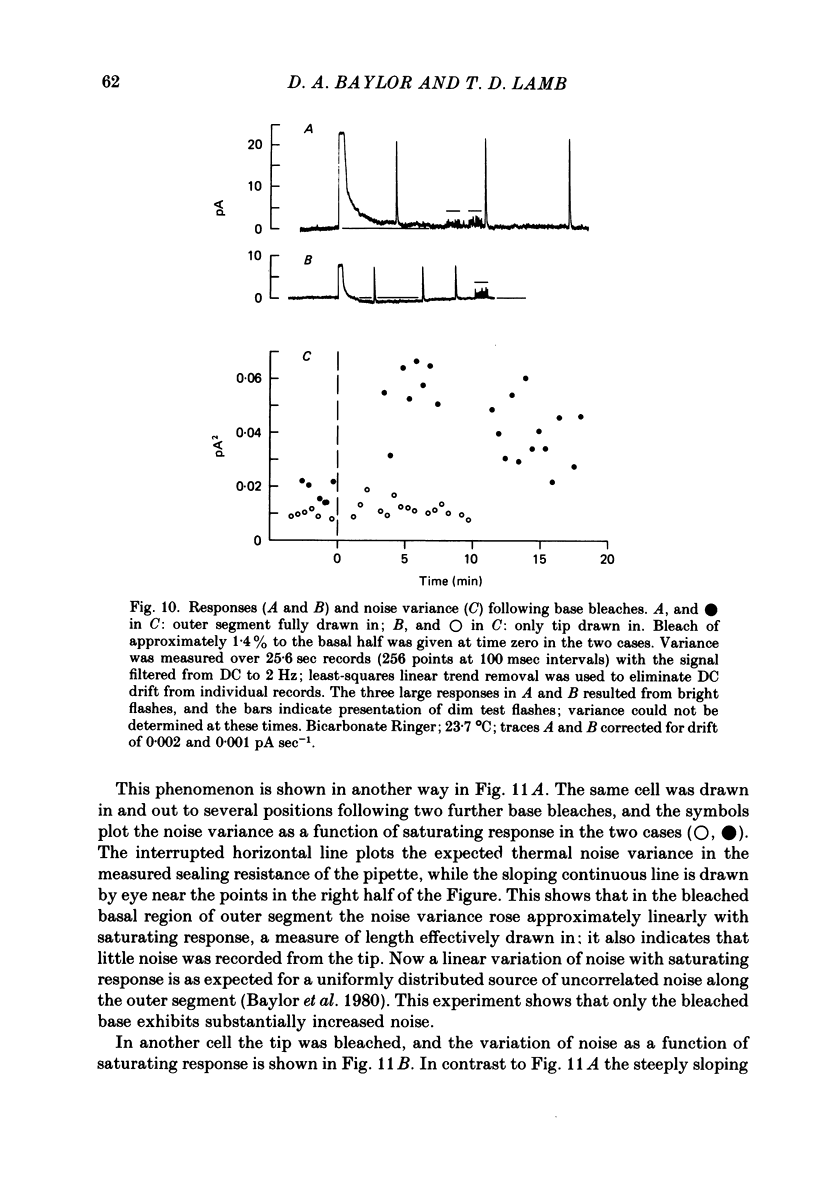
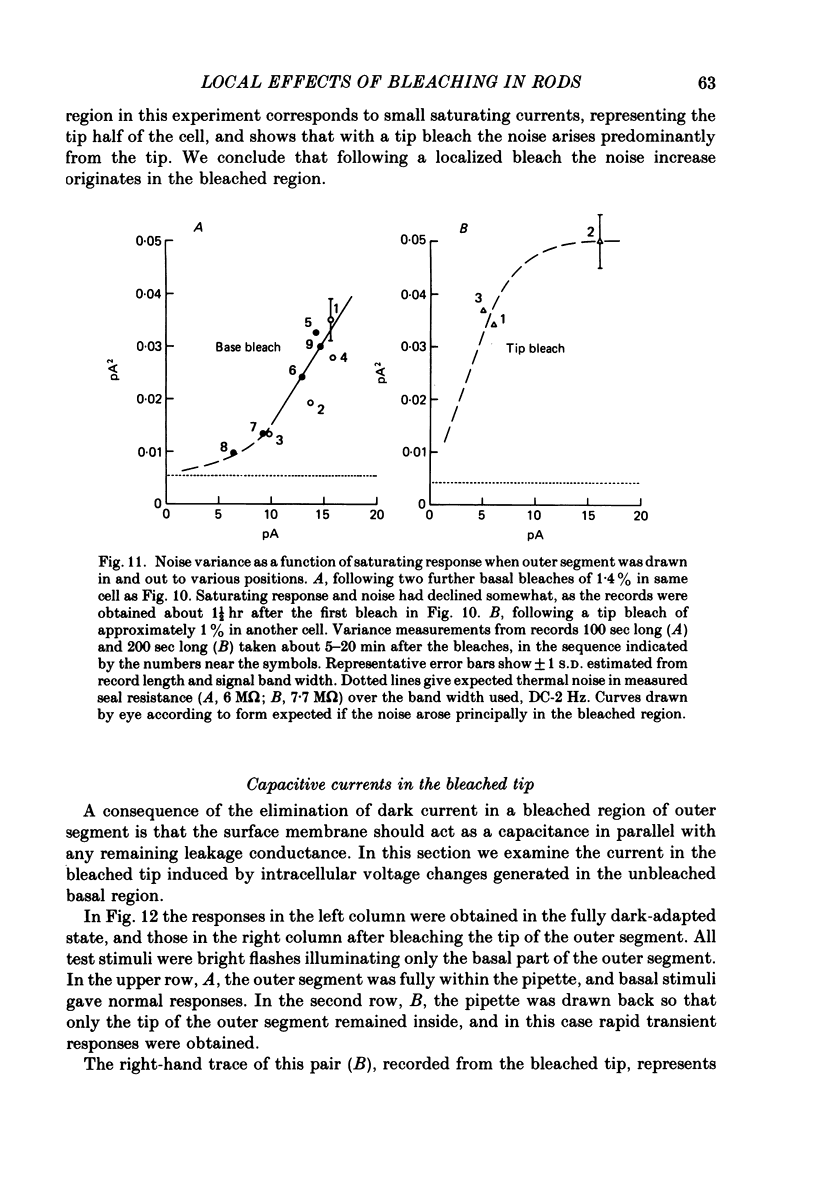
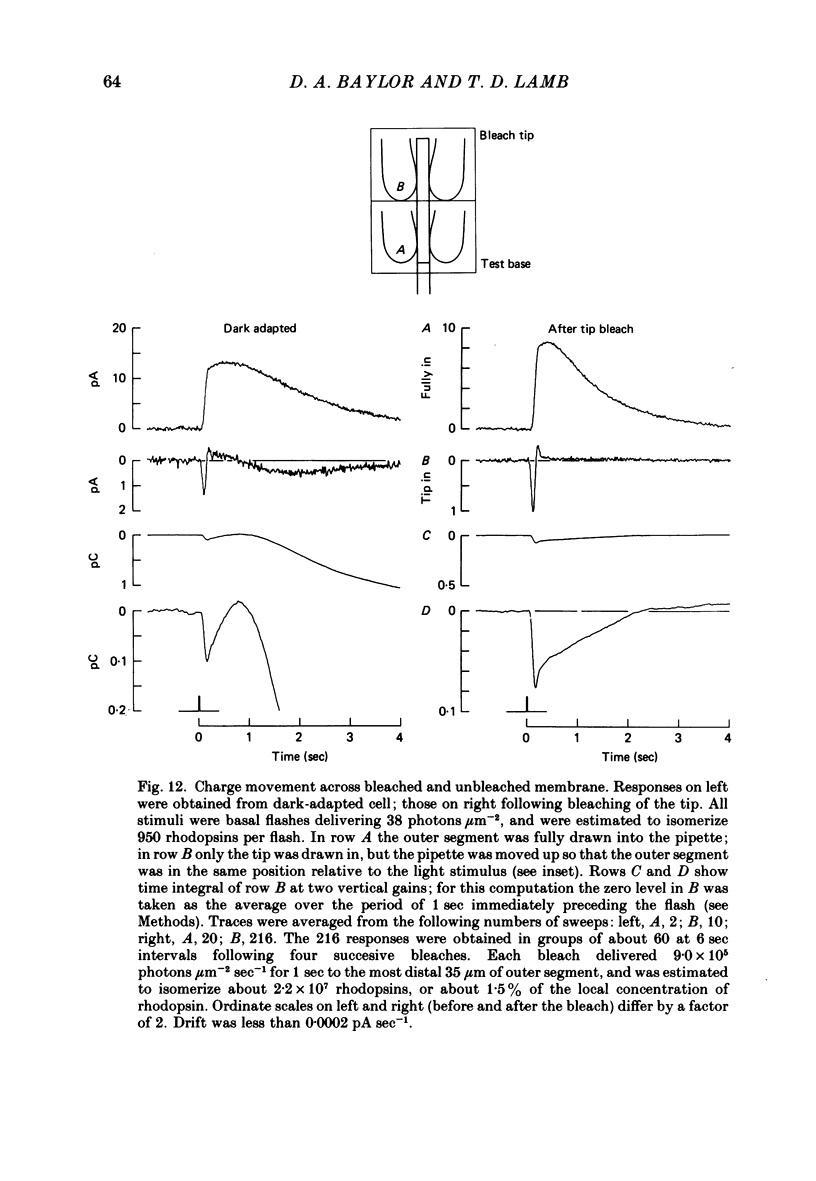





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu R., Barrett E. F. Calcium dependence of evoked transmitter release at very low quantal contents at the frog neuromuscular junction. J Physiol. 1980 Nov;308:79–97. doi: 10.1113/jphysiol.1980.sp013463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K. O., Hemilä S. O. Dark-adaptation of the aspartate-isolated rod receptor potential of the frog retina: threshold measurements. J Physiol. 1979 Feb;287:93–106. doi: 10.1113/jphysiol.1979.sp012648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Adaptation in skate photoreceptors. J Gen Physiol. 1972 Dec;60(6):698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst W., Kemp C. M., Lake N. Studies on the effects of bleaching amphibian rod pigments. IV. Photoresponses recorded intracellularly from axolotl red rods following bleaching flashes. Exp Eye Res. 1978 Jul;27(1):117–127. doi: 10.1016/0014-4835(78)90058-1. [DOI] [PubMed] [Google Scholar]
- Grabowski S. R., Pak W. L. Intracellular recordings of rod responses during dark-adaptation. J Physiol. 1975 May;247(2):363–391. doi: 10.1113/jphysiol.1975.sp010936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä S., Reuter T. Longitudinal spread of adaptation in the rods of the frog's retina. J Physiol. 1981 Jan;310:501–528. doi: 10.1113/jphysiol.1981.sp013564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger W. S. Local stimulation and local adaptation of single isolated frog rod outer segments. Vision Res. 1979;19(4):381–384. doi: 10.1016/0042-6989(79)90098-1. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Spontaneous quantal events induced in toad rods by pigment bleaching. Nature. 1980 Sep 25;287(5780):349–351. doi: 10.1038/287349a0. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg D. R., Brown P. K., Lurie M., Dowling J. E. Visual pigment and photoreceptor sensitivity in the isolated skate retina. J Gen Physiol. 1978 Apr;71(4):369–396. doi: 10.1085/jgp.71.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichi H., Williams T. C. Rhodopsin phosphorylation suggests biochemical heterogeneities of retinal rod disks. J Supramol Struct. 1979;12(4):419–424. doi: 10.1002/jss.400120402. [DOI] [PubMed] [Google Scholar]
- Young R. W. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976 Sep;15(9):700–725. [PubMed] [Google Scholar]


