Abstract
1. Phospholipid vesicles reconstituted with Na—K-ATPase from pig kidney, show slow passive pump-mediated 86Rb fluxes in the complete absence of ATP and phosphate.
2. The Rb fluxes are inhibited in vesicles prepared from enzyme pre-treated with either ouabain or vanadate ions. Rb fluxes through Na—K pumps oriented inside-out or right-side out by comparison with the normal cellular orientation can be distinguished by effects of vanadate on one or both sides of the vesicle.
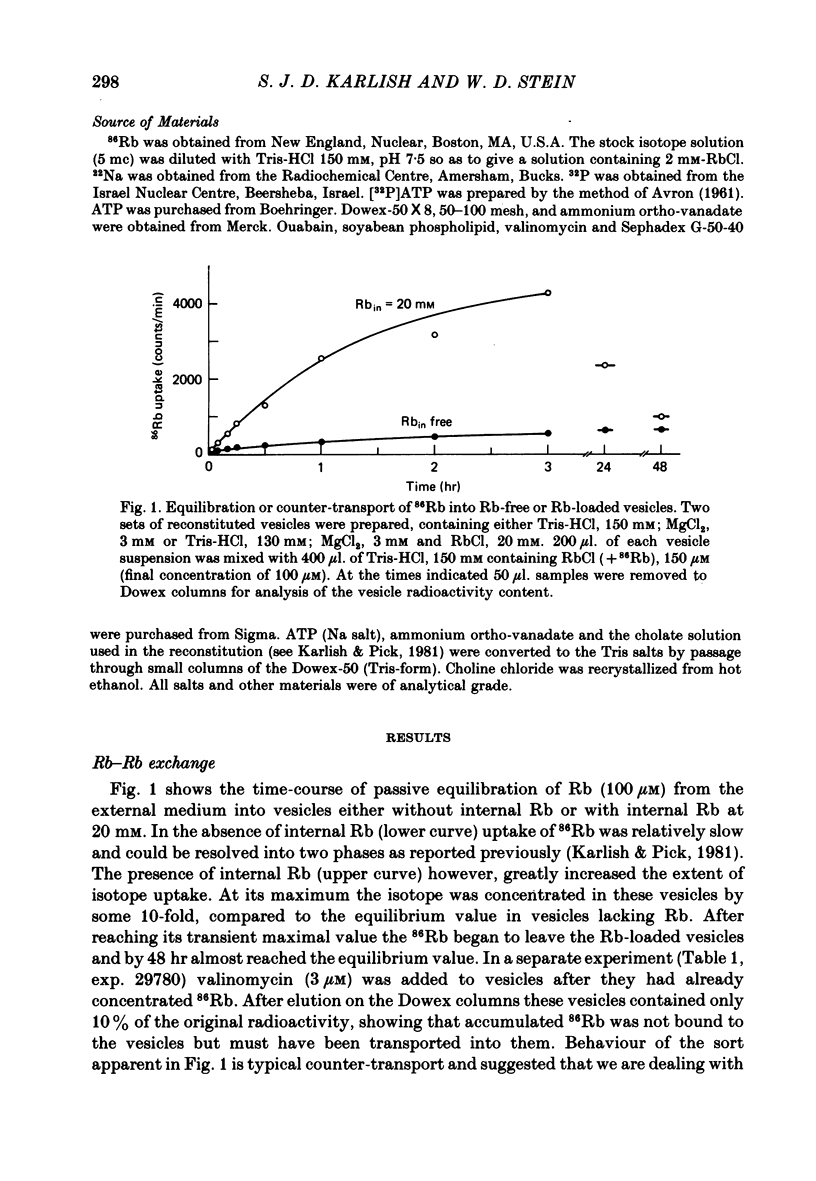
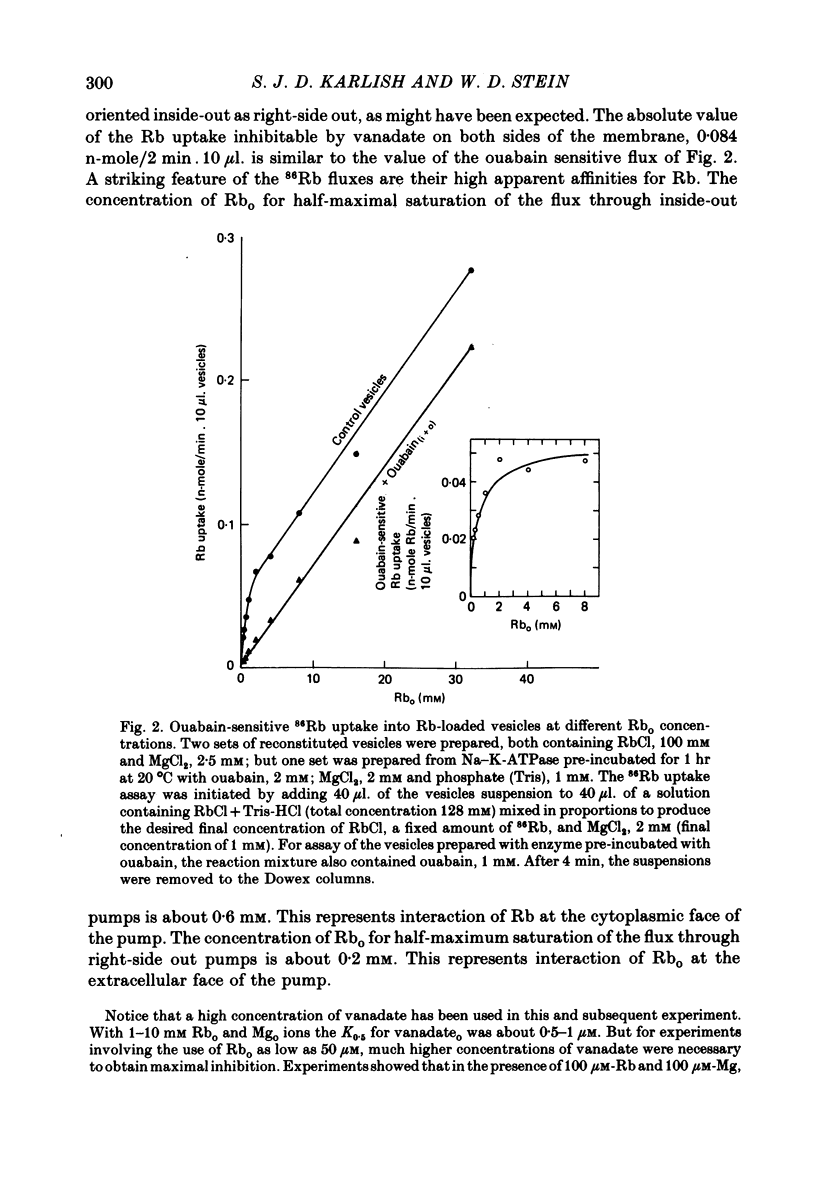
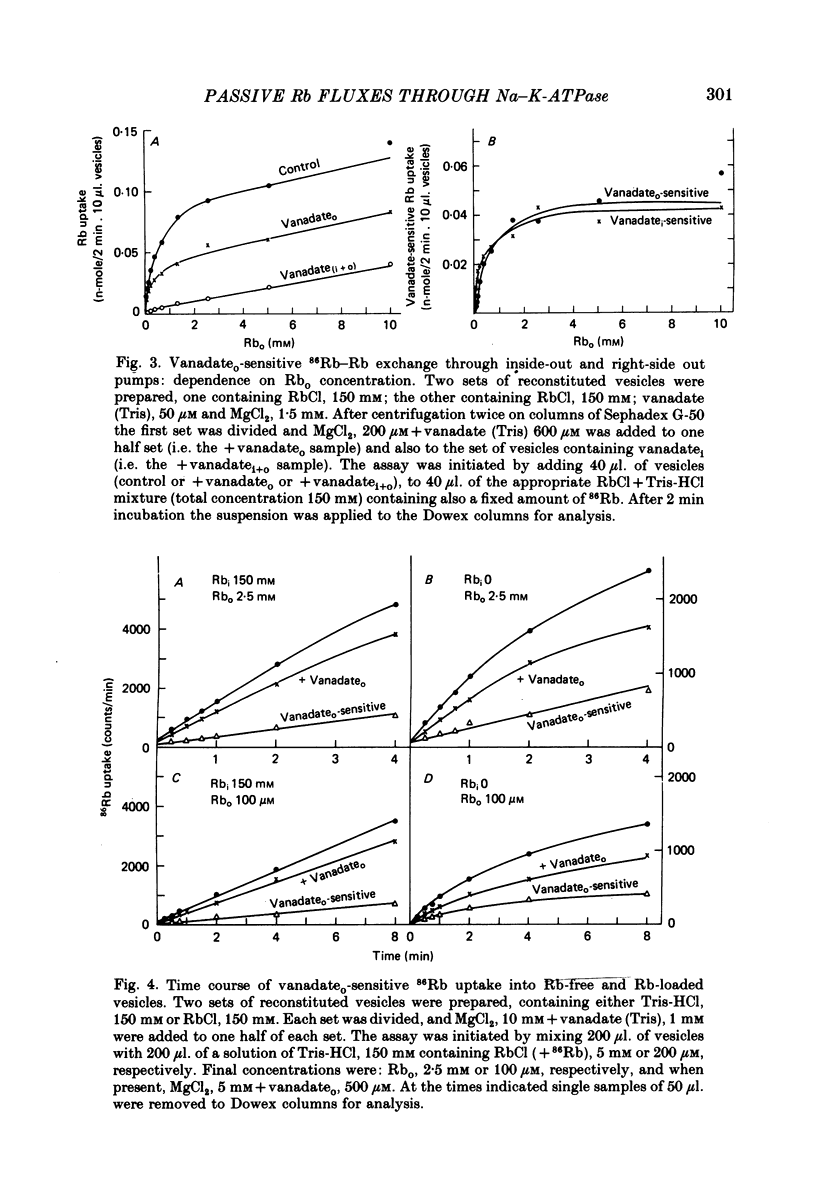
3. 86Rb uptake into Rb-loaded vesicles represents a 86Rb—Rb exchange. The maximal rate of exchange through inside-out and right-side out oriented pumps is equal, suggesting a random arrangement of the pumps across the vesicle membrane. This Rb—Rb exchange is half-saturated on inside-out and right-side out pumps at about 0·6 and 0·2 mM-external Rb respectively.
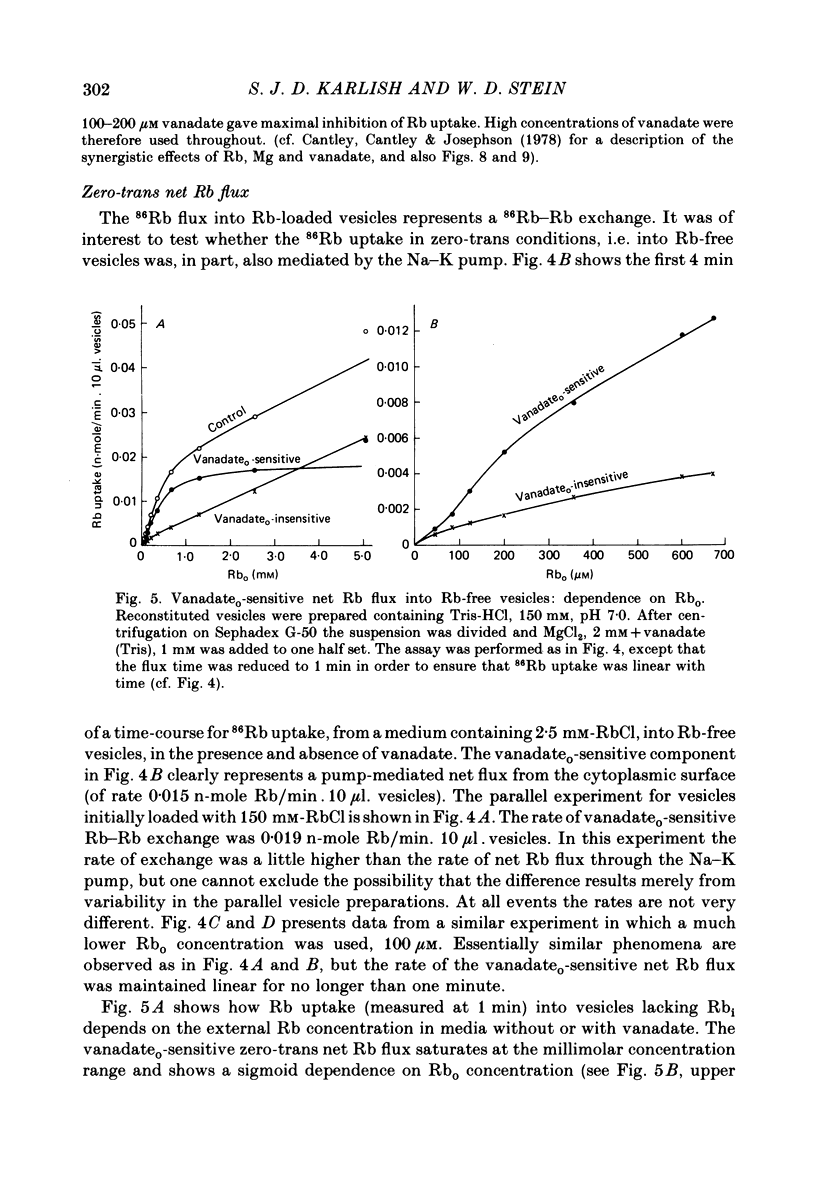
4. 86Rb uptake into Rb-free vesicles represents a net Rb flux. The Rb uptake through inside-out pumps has a maximal rate about equal to the Rb—Rb exchange, half-saturates at an external Rb concentration of roughly 0·5 mM, and shows evidence for co-operativity. Net Rb uptake through right-side out pumps is very slow, and half-saturates at roughly 0·1 mM external Rb.
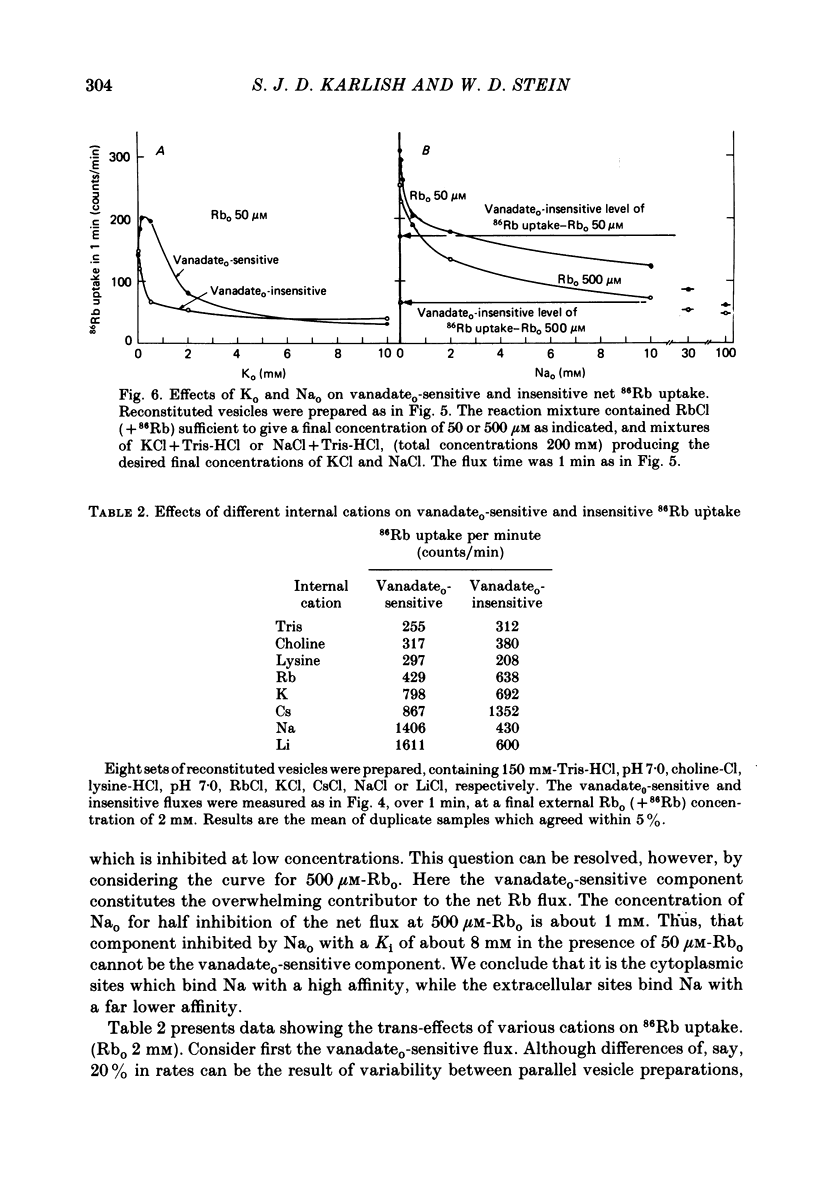
5. K ions at low concentrations in the exterior medium stimulate 86Rb uptake, but at high concentrations, inhibit. Na ions in the exterior medium always inhibit 86Rb uptake. The result suggests that K ions are transported in co-operative fashion together with Rb ions, while Na ions block the Rb fluxes.
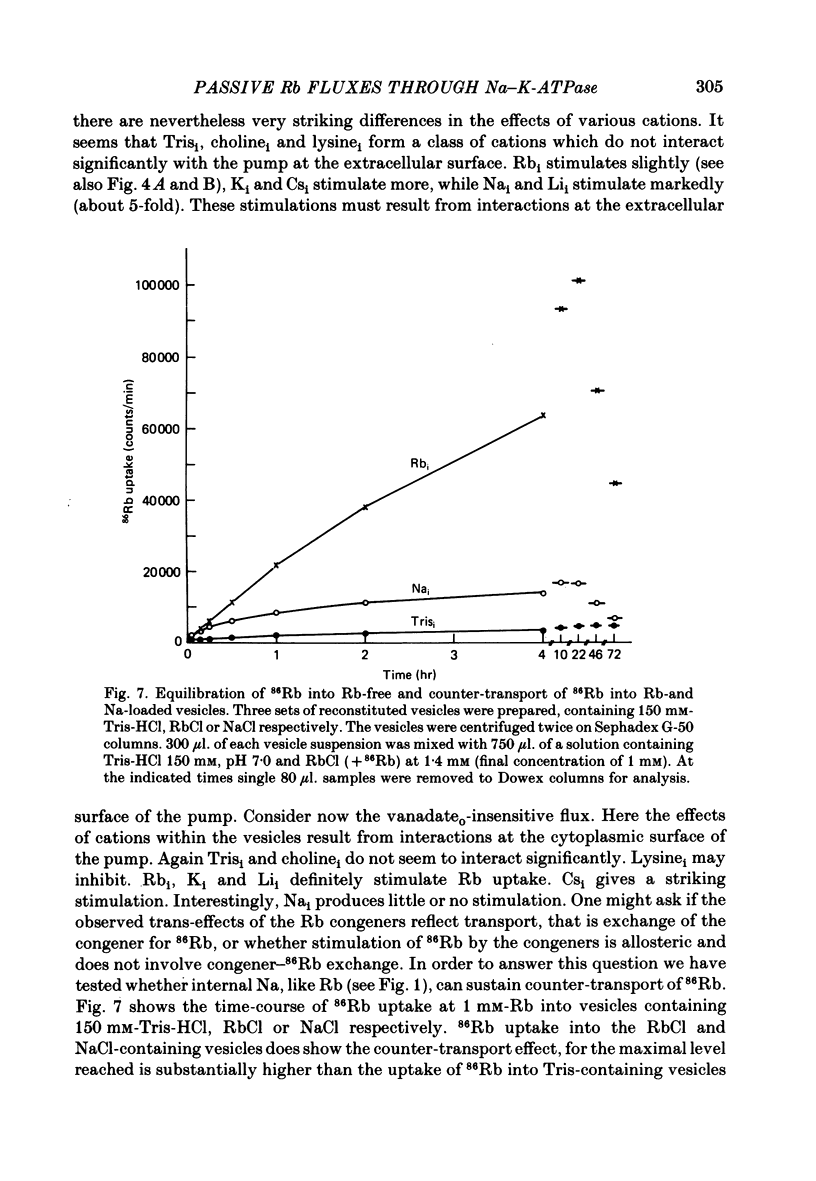
6. The presence of Rb congeners at the vesicle interior raises the 86Rb uptake through inside-out pumps with the decreasing order of effectiveness: Li > Na > Cs > K > Rb. Stimulation by Na ions involves a Rb—Na exchange.
7. Turnover numbers were estimated from parallel measurement of Na/K pump mediated fluxes and amount of covalent phosphoenzyme. In units of moles of ion per mole of phosphoenzyme per second at 20 °C the following values were obtained: ATP-dependent Na—Rb exchange, 43; (ATP+phosphate)-stimulated Rb—Rb exchange, 7. For (ATP+phosphate)-independent fluxes: Rb—Rb exchange 0·25; net Rb uptake 0·15 and Rb—Na exchange 0·65.
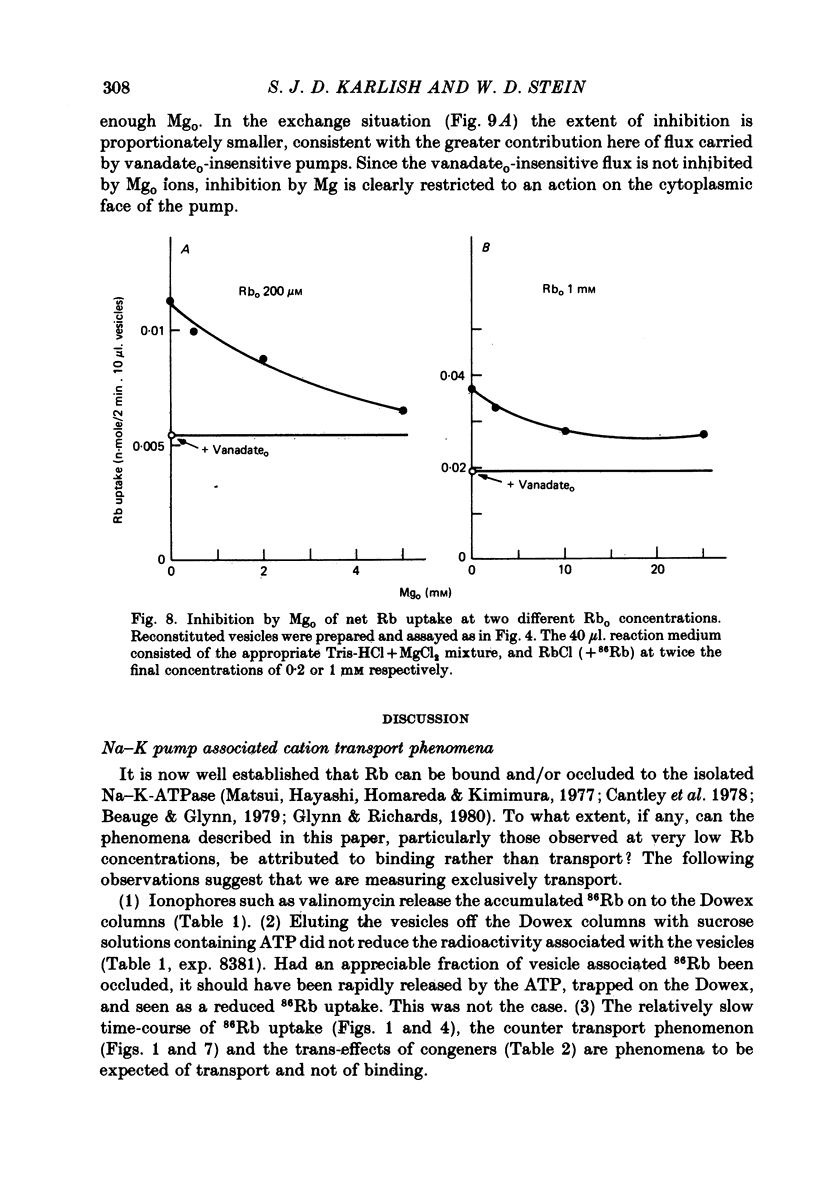
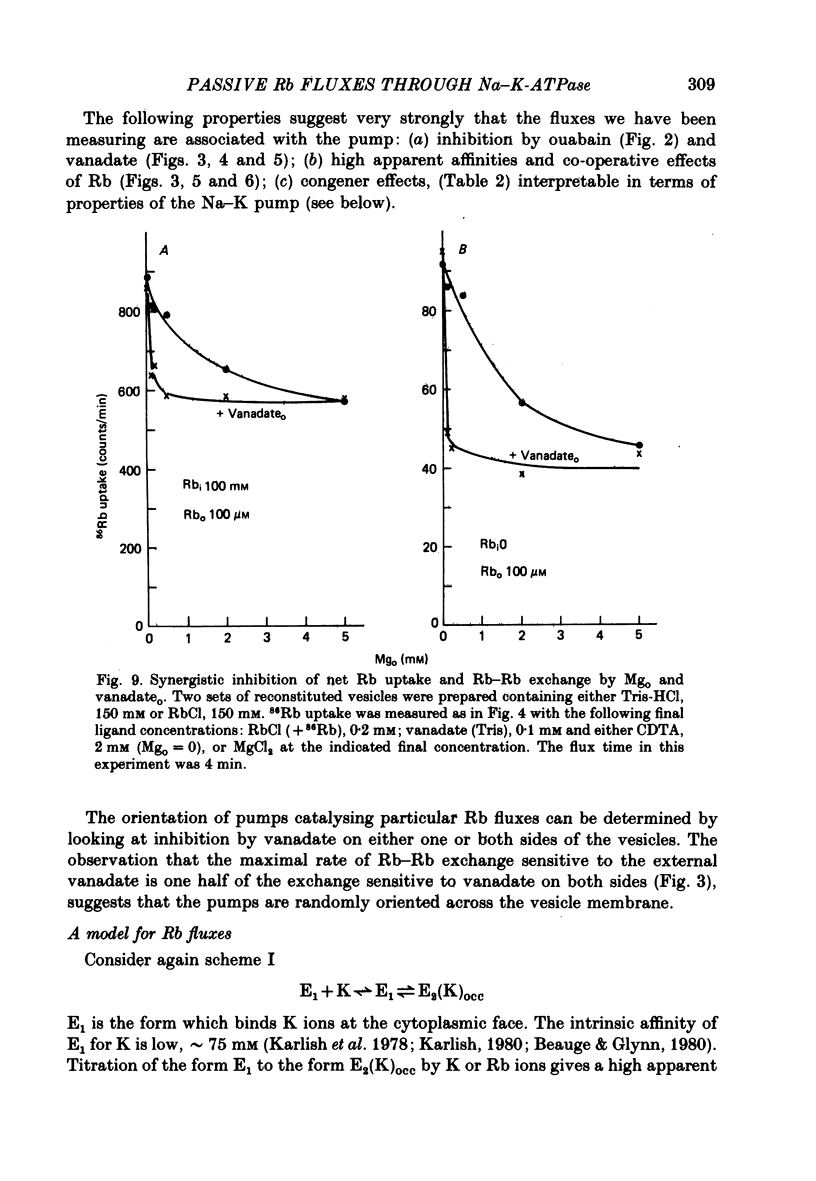
8. Mg ions in the exterior medium inhibited both net and exchange Rb fluxes through inside-out pumps in a manner antagonistic with respect to Rb. Mg and vanadate ions inhibit the Rb fluxes in a synergistic fashion.
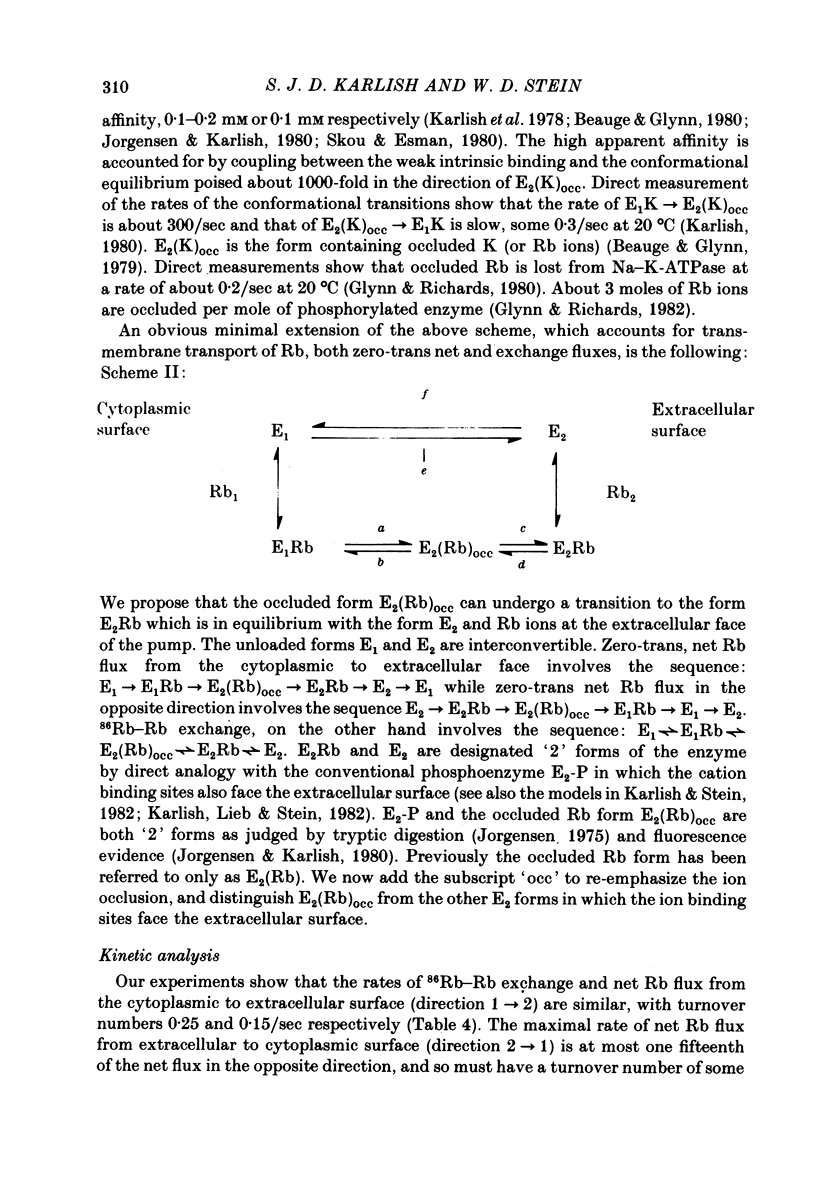
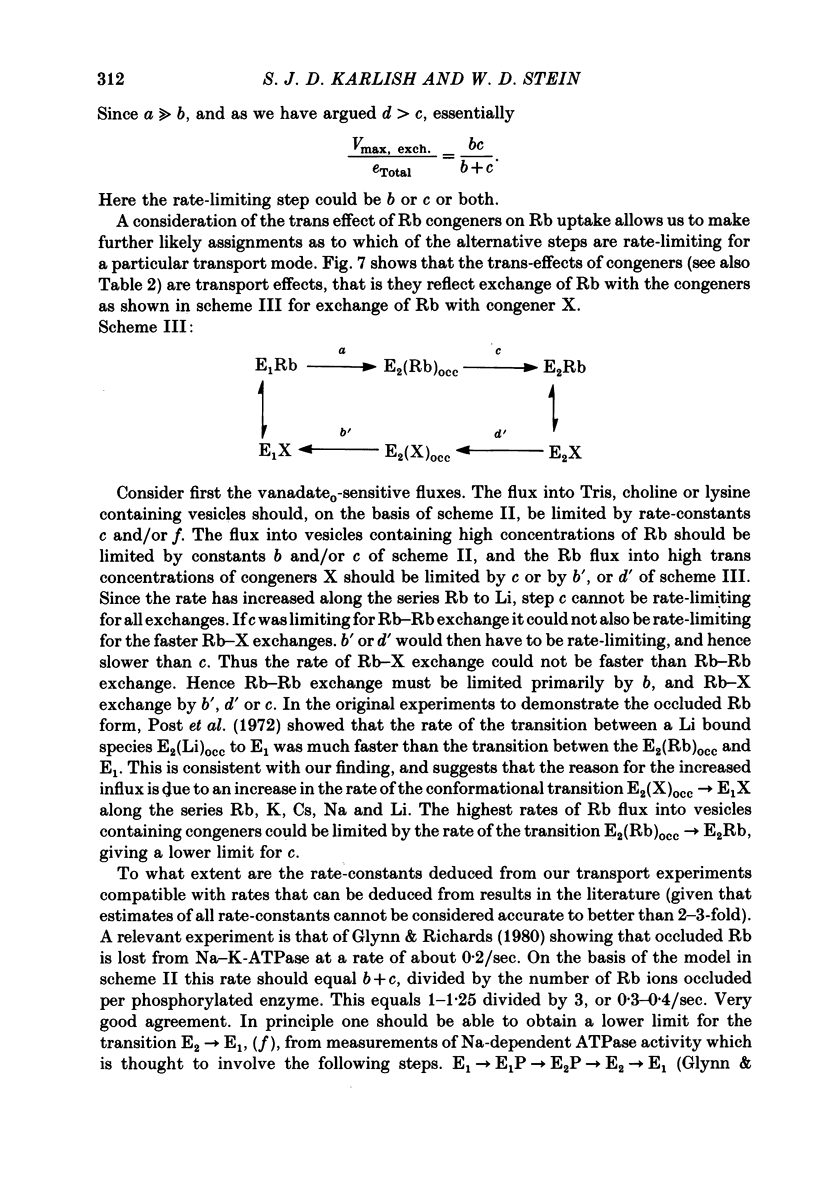
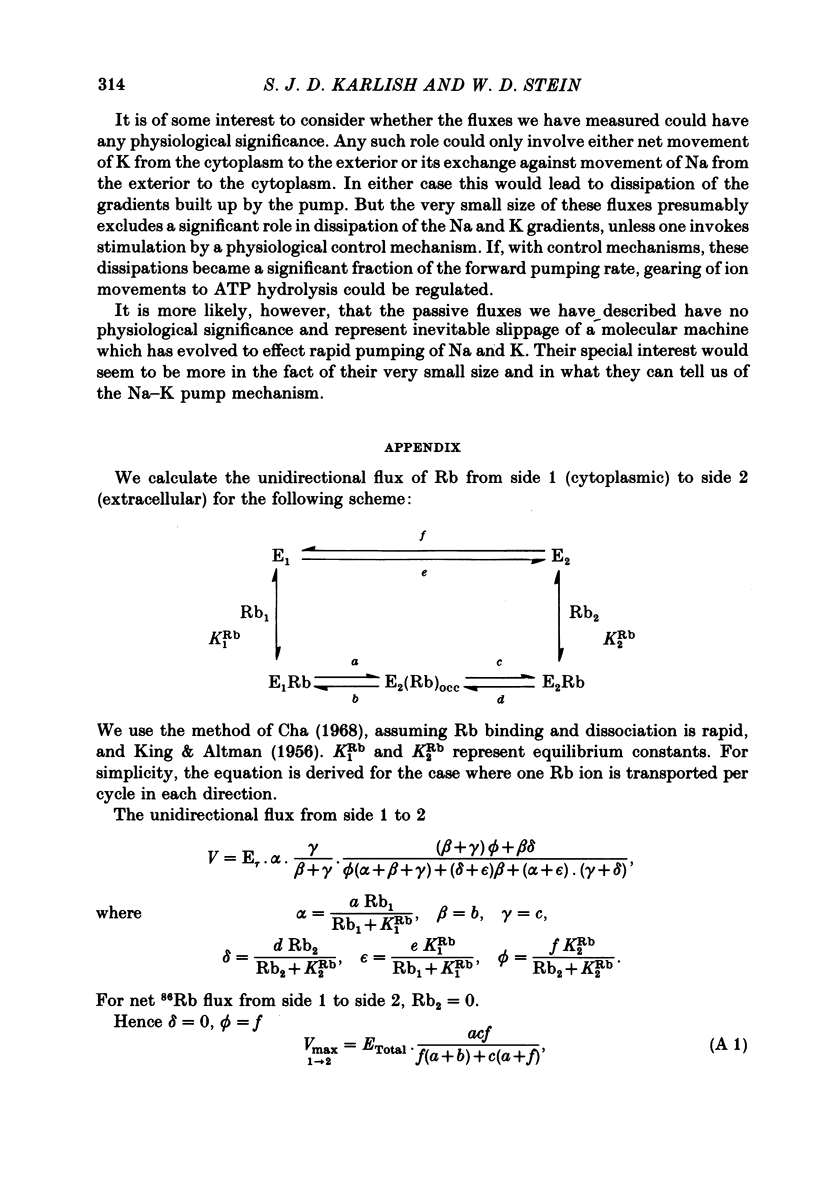
9. The results are interpreted in terms of a model in which net and exchange 86Rb fluxes occur via conformational transitions between form E1 which binds Rb at the cytoplasmic face of the protein, the form E2 (Rb)occ containing occluded Rb ions and a form E2 which binds Rb at the extracellular face of the protein. A kinetic analysis allows us to identify rate-limiting steps of the transport cycle by making use of our transport data in combination with values of rate-constants for conformational transitions observed directly in isolated Na—K-ATPase.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation as a tool for the synthesis of specifically labeled nucleotides. Anal Biochem. 1961 Dec;2:535–543. doi: 10.1016/0003-2697(61)90021-5. [DOI] [PubMed] [Google Scholar]
- Beaugé L. A., Glynn I. M. Occlusion of K ions in the unphosphorylated sodium pump. Nature. 1979 Aug 9;280(5722):510–512. doi: 10.1038/280510a0. [DOI] [PubMed] [Google Scholar]
- Beaugé L. A., Glynn I. M. The equilibrium between different conformations of the unphosphorylated sodium pump: effects of ATP and of potassium ions, and their relevance to potassium transport. J Physiol. 1980 Feb;299:367–383. doi: 10.1113/jphysiol.1980.sp013130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blostein R. Sodium-activated adenosine triphosphatase activity of the erythrocyte membrane. J Biol Chem. 1970 Jan 25;245(2):270–275. [PubMed] [Google Scholar]
- Cantley L. C., Jr, Cantley L. G., Josephson L. A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J Biol Chem. 1978 Oct 25;253(20):7361–7368. [PubMed] [Google Scholar]
- Cantley L. C., Jr, Resh M. D., Guidotti G. Vanadate inhibits the red cell (Na+, K+) ATPase from the cytoplasmic side. Nature. 1978 Apr 6;272(5653):552–554. doi: 10.1038/272552a0. [DOI] [PubMed] [Google Scholar]
- Cha S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J Biol Chem. 1968 Feb 25;243(4):820–825. [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. ATP hydrolysis associated with an uncoupled sodium flux through the sodium pump: evidence for allosteric effects of intracellular ATP and extracellular sodium. J Physiol. 1976 Apr;256(2):465–496. doi: 10.1113/jphysiol.1976.sp011333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P. L. Isolation of (Na+ plus K+)-ATPase. Methods Enzymol. 1974;32:277–290. [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ + K+)-ATPase. VI. Differential tryptic modification of catalytic functions of the purified enzyme in presence of NaCl and KCl. Biochim Biophys Acta. 1977 Apr 1;466(1):97–108. doi: 10.1016/0005-2736(77)90211-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ plus K+ )-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta. 1974 Jul 12;356(1):36–52. doi: 10.1016/0005-2736(74)90292-2. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L., Karlish S. J. Defective conformational response in a selectively trypsinized (Na+ + K+)-ATPase studied with tryptophan fluorescence. Biochim Biophys Acta. 1980 Apr 10;597(2):305–317. doi: 10.1016/0005-2736(80)90108-x. [DOI] [PubMed] [Google Scholar]
- Karlish S. J. Characterization of conformational changes in (Na,K) ATPase labeled with fluorescein at the active site. J Bioenerg Biomembr. 1980 Aug;12(3-4):111–136. doi: 10.1007/BF00744678. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Lieb W. R., Stein W. D. Combined effects of ATP and phosphate on rubidium exchange mediated by Na-K-ATPase reconstituted into phospholipid vesicles. J Physiol. 1982 Jul;328:333–350. doi: 10.1113/jphysiol.1982.sp014267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlish S. J., Pick U. Sidedness of the effects of sodium and potassium ions on the conformational state of the sodium-potassium pump. J Physiol. 1981 Mar;312:505–529. doi: 10.1113/jphysiol.1981.sp013641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlish S. J., Stein W. D. Effects of atp or phosphate on passive rubidium fluxes mediated by Na-K-ATPase reconstituted into phospholipid vesicles. J Physiol. 1982 Jul;328:317–331. doi: 10.1113/jphysiol.1982.sp014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W., Glynn I. M. Conformational transitions between Na+-bound and K+-bound forms of (Na+ + K+)-ATPase, studied with formycin nucleotides. Biochim Biophys Acta. 1978 Jul 7;525(1):252–264. doi: 10.1016/0005-2744(78)90219-x. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W. Tryptophan fluorescence of (Na+ + K+)-ATPase as a tool for study of the enzyme mechanism. Biochim Biophys Acta. 1978 Nov 10;527(1):115–130. doi: 10.1016/0005-2744(78)90261-9. [DOI] [PubMed] [Google Scholar]
- Matsui H., Hayashi Y., Homareda H., Kimimura M. Ouabain-sensitive 42K binding to Na+, K+-ATPase purified from canine kidney outer medulla. Biochem Biophys Res Commun. 1977 Mar 21;75(2):373–380. doi: 10.1016/0006-291x(77)91052-x. [DOI] [PubMed] [Google Scholar]
- Post R. L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6530–6540. [PubMed] [Google Scholar]
- Simons T. J. Potassium: potassium exchange catalysed by the sodium pump in human red cells. J Physiol. 1974 Feb;237(1):123–155. doi: 10.1113/jphysiol.1974.sp010474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. Effects of ATP and protons on the Na : K selectivity of the (Na+ + K+)-ATPase studied by ligand effects on intrinsic and extrinsic fluorescence. Biochim Biophys Acta. 1980 Sep 18;601(2):386–402. doi: 10.1016/0005-2736(80)90543-x. [DOI] [PubMed] [Google Scholar]


