Abstract
The aim of this study is to compare the refractive parameters and visual quality of patients with low to moderate myopia and high myopia before and after transepithelial photorefractive keratectomy(transPRK)to evaluate the efficacy and safety of transPRK in correcting different degrees of myopia, and to explore the impact of the surgery on the visual quality of patients with different spherical equivalent(SER). This study retrospectively included myopic patients who underwent transPRK using the Schwind Amaris excimer laser. The patients were divided into the low to moderate and high myopia groups according to the preoperative spherical equivalent. The subjects’ refractive parameters and visual quality before, at 1, 3, and 6 months after surgery were collected and analysed. The changes in each value before and after surgery (Δ) were compared. At 6 months after surgery, 96.8% (low to moderate myopia group) and 92.3% (high myopia group) of the eyes in both groups achieved uncorrected distance visual acuity (UDVA) of 20/20 or above. At 6 months after surgery, 65.1% of the eyes in the low to moderate myopia group had SER within ± 0.5D, and 95.2% had SER within ± 1.0D. In the high myopia group, 71.8% of the eyes had SER within ± 0.5D, and 97.4% had SER within ± 1.0D. No patient lost one or more lines of corrected distance visual acuity (CDVA) simultaneously after surgery. All patients achieved a CDVA of 20/20 after surgery. There were significant differences in △corneal higher-order aberrations at 6.0 mm pupil(C.HOA), △corneal spherical aberration at 6.0 mm pupil(C.Sph), and △corneal coma aberration at 6.0 mm pupil(C.Coma) between the two groups (P < 0.05). In comparison, there were no significant differences in △corneal trefoil aberration at 6.0 mm pupil(C.Tre), △and the strehl ratio(SR) between the two groups (P > 0.05). There were significant differences in △central corneal thickness (CCT), △anterior surface asphericity of the cornea (Q), △eccentricity (e) and △corneal curvature (K1, K2, and Km) between the two groups (P < 0.001). In the low to moderate myopia group, △SER was positively correlated with △C.HOA and △C.Sph (P < 0.01), and △SER was positively correlated with △C.Coma at 6 months (P < 0.01); there was no correlation between △SER and △C.Tre and △SR (P > 0.05). In the high myopia group, △SER was positively correlated with △C.HOA at 6 months (P < 0.05); △SER was positively correlated with △C.Sph and △SR at 3 and 6 months (P < 0.05). There was no correlation between △SER and △C.Coma or △C.Tre (P > 0.05). This study demonstrates that transPRK can safely and effectively correct the UDVA and refractive status of patients with low to moderate and high myopia. Compared with patients with low to moderate myopia, patients with high myopia have more increases in C.HOA, C.Sph, and C.Coma at the same time point after surgery.△SER is positively correlated with △C.HOA after surgery. Among the three aberrations, the correlation between △SER and △C.Sph is the strongest in the high myopia group simultaneously.
Keywords: TransPRK, Diopter, Visual quality
Subject terms: Refractive errors, Outcomes research
Introduction
Myopia is the most common refractive error and is one of the leading causes of visual impairment worldwide1,2. Due to its high prevalence, myopia has become a global public health problem3. Most studies have now reached a consensus on myopia and high myopia: myopia is defined as Spherical Equivalent(SER) of at least − 0.50 D, and high myopia is defined as SER of at least − 6.00 D4. (SER = Diopter Sphere + 1/2 Diopter Cylinder).
With the development of science and technology, corneal refractive surgery has been developing rapidly. Its safety, effectiveness and accuracy have been continuously improving, and it has provided patients with multiple surgical options, including photorefractive keratectomy (PRK), femtosecond laser-assisted in situ keratomileusis (FS-LASIK) and small incision lenticule extraction (SMILE).PRK has been an established and safe surface ablation technique for decades, and transepithelial photorefractive keratectomy(transPRK)is a modification and alternative to traditional PRK. TransPRK uses an excimer laser to perform corneal epithelial cutting first, then continues to use the same laser to cut the underlying stroma. It takes less time, reduces postoperative discomfort, and enables faster vision recovery5–9, with significantly enhanced safety. Compared to other procedures, transPRK offers greater flexibility in corneal ablation and greater biomechanical stability of the patient’s cornea postoperatively10,11 without the complications associated with flaps12–15. It is also thought to have less aberration due to greater preservation of corneal integrity16,17. As a result, transPRK currently serves as one of the first choices of corneal refractive surgeries for patients. It also has limitations compared to other procedures, such as a greater likelihood of corneal haze18,19.
In recent years, the issue of visual quality has received increasing attention. After corneal refractive surgery, most patients achieve satisfactory uncorrected distance visual acuity(UDVA). However, a small number of patients reported that their subjective feelings after the surgery were not satisfactory, such as glare, halo, double vision and poor night vision, which were related to the inevitable increase of high-order aberrations during the surgery, resulting in the deterioration of visual quality20. High-order aberrations include spherical aberration, coma aberration, and trefoil aberration, etc., which significantly impact visual quality21. The point spread function (PSF) describes the shape of a very distant target point light source imaged on the retina. The specific value is reflected by the strehl ratio (SR). The higher the SR value, the better the retinal imaging quality and visual quality. We know that patients with high myopia can have refractive progression after surgery due to the elongation of the eye axis. Whether they can have satisfactory long-term therapeutic effects after surgery and whether the visual quality will be lower than that of patients with low or moderate myopia due to excessive corneal ablation are issues that many patients with high myopia consult and worry about before surgery.
Currently, most of the related studies are focused on comparing the clinical parameters before and after surgery for different or the same surgical methods. In this study, we further divided the patients into low, moderate and high myopia groups, and compared the changes in visual quality parameters and other refractive parameters between the groups. It also studies the correlation between the changes in clinical parameters and the changes in refractive power. At the same time, this study also incorporates astigmatism vector analysis. Through more comprehensive comparisons, this study aims to evaluate the efficacy and safety of transPRK in correcting different degrees of myopia and to explore the impact of the surgery on the visual quality of patients with different diopters. The results can be more beneficial for setting surgical parameters for patients with different diopters, helping patients achieve individualised treatment plans and obtain more efficient visual quality after surgery.
Methods
Study design
This was a retrospective study using a non-randomised controlled design. The study included patients with myopia (with or without astigmatism) who underwent transPRK with the Schwind Amaris excimer laser from February 2023 to December 2024 at the Second Affiliated Hospital of Dalian Medical University. The patients were divided into a low-moderate myopia group (SER ≤ −6.00 D) and a high-myopia group (SER > −6.00 D) according to the preoperative spherical equivalent. The total of 63 eyes in the low-moderate myopia group and 39 eyes in the high-myopia group were included. The subjects’ refractive parameters and visual quality were collected and analysed preoperatively and at 1, 3 and 6 months postoperatively, including uncorrected distance visual acuity(UDVA), SER, intraocular pressure (IOP), anterior surface asphericity of the cornea (Q-value), eccentricity (e-value), central corneal thickness (CCT), corneal curvature (K1, K2, Km), corneal higher-order aberrations at 6.0 mm pupil (C.HOA), corneal spherical aberration at 6.0 mm pupil (C.Sph), corneal coma aberration at 6.0 mm pupil (C.Coma), corneal trefoil aberration at 6.0 mm pupil (C.Tre) and strehl ratio (SR), as well as comparing the amount of postoperative versus preoperative change (Δ) in each of these values. All patients agreed to participate in the study and signed an informed consent form. The clinical examinations involved in this study were by the Declaration of Helsinki and approved by the Ethics Committee of the Second Affiliated Hospital of Dalian Medical University.
Inclusion criteria
Patients aged over 18 years;
SER does not exceed − 8.00D while Cylindrical diopter does not exceed 4.00D;
Spherical diopter does not change by more than 1.00D in the past two years and 0.50D in six months; Cylindrical diopter does not change by more than 2.00D in the past year;
Postoperative residual thickness of the corneal stroma was greater than or equal to 350 μm;
Agree to participate in the study and sign the informed consent form.
Exclusion criteria
The corneal topography shows characteristics of keratoconus or tends to corneal dilation;
Preoperative corrected distance visual acuity of less than 20/25 or pathological myopia or posterior scleral pathology;
Previous history of ophthalmic surgery and other ocular diseases (e.g. glaucoma, severe dry eye, active ocular diseases, corneal dystrophy or degeneration, retinal diseases, endophthalmitis, refractive media clouding, etc.);
Associated systemic diseases (e.g. rheumatoid arthritis, diabetes mellitus, etc.);
Pregnant and lactating women;
Those patients without regular follow-up.
Preoperative preparation and basic examination
All patients received a complete ophthalmological examination before surgery. These included slit lamp examination, UDVA and corrected distance visual acuity (CDVA) examination, post-dilation refraction examination, intraocular pressure examination, corneal thickness measurement, corneal topography examination, corneal/refractive analyser(OPD) examination, fundus examination, Optical Coherence Tomography(OCT) examination, dry eye examination, corneal endothelial microscopy examination, anterior chamber depth examination and lacrimal canal flushing. Sodium hyaluronate eye drops, levofloxacin eye drops, and Pralofen eye drops were used in both eyes starting 3 days before surgery, all 4 times a day, with an interval of 5–10 min between the two drops. The surgery requires that soft contact lenses be discontinued for one week, rigid gas-permeable contact lenses be removed for three weeks, and night-wear rigid corneal orthokeratology lenses be removed for over three months. Among them: a visual acuity examination was carried out using the international standard logarithmic distance visual acuity chart (GB11533-2011) for both eyes of the subjects for uncorrected distance visual acuity and best-corrected distance visual acuity, with a detection distance of 5 m and converted to logarithmic of the minimum angle of resolution (LogMAR); IOP was measured using a non-contact tonometer (NT-530P, NIDEK). Measurements including Q-value, e-value, and C.HOA, C.Sph, C.Coma, C.Tre and SR were made using a corneal/refractive analyser (OPD-Scan III, NIDEK), and measurements were taken using a LENSTAR (HAAG-STREIT, LS 900) to measure corneal thickness and corneal curvature.
Surgical procedures
The same experienced clinician was used to determine the surgical volume and perform the surgical procedures. All procedures were performed in the same room using the same Amaris excimer laser device (laser treatment frequency: 500 Hz, spot diameter: 0.54 mm, eye tracking frequency: 1050 Hz), and the mode of choice was Smart Pulse Technology-guided aberration-balanced. Surface anaesthesia was administered with 0.01% proparacaine hydrochloride eye drops. The patients were asked to focus on the fixation lamp to centre the ablation zone. According to a population-based epithelium-thickness profile, the ablation plan utilized 55 μm centrally, and 65 μm peripherally. Eye movements throughout the ablation were compensated by static and dynamic cyclotorsion corrections (SCC and DCC). After laser ablation, the eyes were rinsed with a cooled balanced salt solution, and a bandage contact lens was placed over the corneal surface. The lens was reviewed one week later to confirm good corneal epithelial growth and was removed.
The design and selection of the light zone in this study are as follows: (1) −1D ≤ SER ≤ −2D, the light zone is ≥ 7.3 mm; (2) −2D < SER ≤ −3D, the light zone is ≥ 6.8 mm; (3) −3D < SER ≤ −8D, the light zone is ≥ 6.3 mm. When the patient’s preoperative refractive error is significant, a relatively minor light zone is selected to save tissue and ensure that the cornea has sufficient remaining stromal bed thickness. The equipment calculates the transition zone automatically.
Postoperative care and follow-up
Slit lamp examination was performed at 1 week, 1 month, 3 months, 6 months and 1 year after surgery to observe the healing of the corneal epithelium and the presence of subepithelial clouding of the cornea, as well as visual acuity examination, optometry examination, intraocular pressure examination, OPD and LENSTAR examination (the specific contents of the examination were the same as those of the preoperative period). Wear an eye mask to sleep and avoid strenuous exercise within one week after surgery; avoid contact with dirt in the eye within two weeks after surgery; wear sunglasses under intense ultraviolet light exposure within six months after surgery. After the first day of postoperative review, start to use tobramycin and dexamethasone eye drops (replaced by fluorometholone eye drops after 1 week), levofloxacin eye drops, bromonidine tartrate eye drops, ganciclovir ophthalmic gel, and sodium hyaluronate eye drops as prescribed by the doctor. The interval between two eye drops was 5–10 min.
Statistical analysis
Statistical analysis was performed using SPSS version 24.0. Data were tested for normality using the Shapiro-Wilk test. Continuous variables were described as mean ± standard deviation. When comparing the general information between the two groups, an independent samples t-test was used to conform to normal distribution, non-normally distributed data were tested using the nonparametric Mann-Whitney U-test, and the chi-square test was used to analyse the gender differences between the two groups. When analysing the differences between each data set’s preoperative and postoperative values, the ANOVA test was used if the data conformed to normal distribution; the Friedman test was used for non-normally distributed data. When analysing the differences in the preoperative and postoperative changes in the data between groups at different time points, the independent samples t-test was used if the changes conformed to normal distribution; the nonparametric Mann-Whitney U test was used if they conformed to nonnormal distribution. For correlation analysis between the preoperative and postoperative change and ΔSER, Pearson’s correlation coefficient was used for normally distributed data, and Spearman’s correlation coefficient was used for non-normally distributed data. P-values < 0.05 were considered to be statistically significant. All corneal aberration results were expressed using root mean square (RMS) values. Vector analyses were performed, and single-angle polar plots were constructed using AstigMATIC software.
Results
Demographic data and preoperative baseline characteristics
This study involved 102 eyes of 51 patients. Among them, 63 eyes belonged to the group with low to moderate myopia, with the average age of the patients being 26.5 ± 8.3 (ranging from 18 to 43 years old), including 15 males and 21 females. The other 39 eyes belonged to the group with high myopia, with the average age of the patients being 26.2 ± 7.2 (ranging from 18 to 38 years old), including 10 males and 14 females. There was no statistical difference between the two groups in gender, mean age, Q-value, e-value, C.HOA, C.Sph, C.Coma, C.Tre, CCT, K1, K2, Km, and SR (P > 0.05). In contrast, the differences in UDVA (LogMAR), SER, and IOP were statistically significant (P < 0.05), and the values were higher in the high myopic group. CDVA could reach 0 (LogMAR) in both groups. The relevant data are shown in Table 1.
Table 1.
Demographic data and preoperative baseline characteristics.
| low to moderate myopia group n = 63 |
High myopia group n = 39 |
P | |
|---|---|---|---|
| Age | 26.50 ± 8.32 | 26.17 ± 7.20 | 0.982 |
| Gender | |||
| Man | 15 (41.7%) | 10 (41.7%) | 1.000 |
| Female | 21 (58.3%) | 14 (58.3%) | |
| UDVA(logMAR) | 0.93 ± 0.31 | 1.15 ± 0.25 | 0.000* |
| SER(D) | −4.24 ± 1.09 | −7.05 ± 0.58 | 0.000* |
| IOP(mmHg) | 15.80 ± 2.18 | 16.70 ± 2.74 | 0.023* |
| Q | −0.13 ± 0.14 | −0.17 ± 0.14 | 0.154 |
| e | 0.29 ± 0.25 | 0.36 ± 0.25 | 0.071 |
| CCT(µm) | 535.73 ± 29.14 | 544.62 ± 24.34 | 0.115 |
| K1(D) | 42.56 ± 1.63 | 43.03 ± 1.64 | 0.159 |
| K2(D) | 43.86 ± 1.70 | 44.39 ± 1.56 | 0.133 |
| Km(D) | 43.20 ± 1.64 | 43.79 ± 1.56 | 0.074 |
| C.HOA(µm) | 0.40 ± 0.08 | 0.38 ± 0.05 | 0.149 |
| C.Sph(µm) | 0.27 ± 0.08 | 0.26 ± 0.07 | 0.546 |
| C.Coma(µm) | 0.23 ± 0.08 | 0.23 ± 0.08 | 0.962 |
| C.Tre(µm) | 0.14 ± 0.07 | 0.14 ± 0.05 | 0.933 |
| SR | 0 | 0 | 0.131 |
*p < 0.05 was considered statistically significant. Values are presented as mean ± standard deviation. UDVA: uncorrected distance visual acuity; logMAR: logarithm of the minimum angle of resolution; SER: spherical equivalent; IOP: intraocular pressure; Q: anterior surface asphericity of the cornea; e: eccentricity; CCT: central corneal thickness; K1, K2, Km: corneal curvature; C.HOA: corneal higher-order aberrations at 6.0 mm pupil; C.Sph: corneal spherical aberration at 6.0 mm pupil; C.Coma: corneal coma aberration at 6.0 mm pupil; C.Tre: corneal trefoil aberration at 6.0 mm pupil; SR: strehl ratio.
Comparison of parameters before and after surgery
As shown in Tables 2 and 3, all the statistical data showed significant differences before and after the surgery (P < 0.001). The postoperative values of UDVA (LogMAR), SER, IOP, CCT, K1, K2, and Km decreased, while those of C.HOA, C.Sph, C.Coma, C.Tre, and SR increased. Q changed from negative to positive, and e changed from positive to negative. At the same postoperative time points, the values of IOP, SR, CCT, K1, K2, and Km in the high myopia group were lower than those in the low myopia group, while the values of C.HOA, C.Sph, C.Coma, Q, and e in the high myopia group were higher than those in the low myopia group. The values of C.Tre were similar in both groups.
Table 2.
Comparison of pre- and post-surgical parameters in the low and moderate myopia group.
| Low to moderate myopia group | pre-operation | 1 month | 3 months | 6 months | F | P |
|---|---|---|---|---|---|---|
| UDVA(logMAR) | 0.93 ± 0.310 | 0.01 ± 0.02 | 0 ± 0 | 0.00 ± 0.02 | 135.459 | 0.000* |
| SER(D) | −4.24 ± 1.09 | 0.39 ± 0.67 | 0.36 ± 0.55 | 0.14 ± 0.53 | 123.561 | 0.000* |
| IOP(mmHg) | 15.80 ± 2.18 | 11.75 ± 2.71 | 11.13 ± 2.55 | 11.40 ± 2.51 | 88.626 | 0.000* |
| Q | −0.13 ± 0.14 | 0.17 ± 0.36 | 0.34 ± 0.42 | 0.29 ± 0.34 | 53.191 | 0.000* |
| e | 0.29 ± 0.25 | −0.21 ± 0.48 | −0.43 ± 0.46 | −0.44 ± 0.43 | 53.191 | 0.000* |
| CCT(µm) | 535.73 ± 29.14 | 444.49 ± 41.08 | 446.48 ± 41.11 | 456.73 ± 42.71 | 79.425 | 0.000* |
| K1(D) | 42.56 ± 1.63 | 38.92 ± 2.06 | 38.91 ± 2.04 | 39.13 ± 1.84 | 54.216 | 0.000* |
| K2(D) | 43.86 ± 1.70 | 39.90 ± 2.26 | 39.76 ± 2.21 | 39.98 ± 2.06 | 56.871 | 0.000* |
| Km(D) | 43.20 ± 1.64 | 39.40 ± 2.15 | 39.33 ± 2.11 | 39.55 ± 1.94 | 56.492 | 0.000* |
| C.HOA(µm) | 0.40 ± 0.08 | 0.560 ± 0.11 | 0.597 ± 0.10 | 0.65 ± 0.10 | 65.458 | 0.000* |
| C.Sph(µm) | 0.27 ± 0.08 | 0.40 ± 0.10 | 0.43 ± 0.10 | 0.45 ± 0.08 | 42.023 | 0.000* |
| C.Coma(µm) | 0.23 ± 0.08 | 0.40 ± 0.10 | 0.35 ± 0.10 | 0.41 ± 0.12 | 53.516 | 0.000* |
| C.Tre(µm) | 0.14 ± 0.07 | 0.21 ± 0.09 | 0.22 ± 0.09 | 0.23 ± 0.10 | 29.000 | 0.000* |
| SR | 0 | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.03 ± 0.01 | 66.743 | 0.000* |
*p < 0.05 was considered statistically significant. Values are presented as mean ± standard deviation. UDVA: uncorrected distance visual acuity; logMAR: logarithm of the minimum angle of resolution; SER: spherical equivalent; IOP: intraocular pressure; Q:anterior surface asphericity of the cornea; e: eccentricity; CCT: central corneal thickness; K1, K2, Km: corneal curvature; C.HOA: corneal higher-order aberrations at 6.0 mm pupil; C.Sph: corneal spherical aberration at 6.0 mm pupil; C.Coma: corneal coma aberration at 6.0 mm pupil; C.Tre: corneal trefoil aberration at 6.0 mm pupil; SR: strehl ratio.
Table 3.
Comparison of parameters before and after surgery in the high myopia group.
| High myopia group | pre-operation | 1 month | 3 months | 6 months | F | P | |
|---|---|---|---|---|---|---|---|
| UDVA(logMAR) | 1.15 ± 0.25 | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.01 ± 0.04 | 112.254 | 0.000* | |
| SER(D) | −7.1 ± 0.58 | −0.05 ± 0.68 | −0.17 ± 0.68 | −0.15 ± 0.53 | 78.107 | 0.000* | |
| IOP(mmHg) | 16.70 ± 2.74 | 11.04 ± 3.21 | 10.54 ± 2.01 | 10.10 ± 1.71 | 60.132 | 0.000* | |
| Q | −0.17 ± 0.14 | 0.57 ± 0.45 | 0.76 ± 0.41 | 0.73 ± 0.26 | 60.421 | 0.000* | |
| e | 0.36 ± 0.25 | −0.66 ± 0.40 | −0.85 ± 0.22 | −0.82 ± 0.16 | 52.046 | 0.000* | |
| CCT(µm) | 544.62 ± 24.34 | 404.59 ± 26.41 | 410.30 ± 26.08 | 416.04 ± 29.48 | 247.715 | 0.000* | |
| K1(D) | 43.03 ± 1.64 | 37.34 ± 1.70 | 37.31 ± 1.60 | 37.30 ± 1.39 | 119.895 | 0.000* | |
| K2(D) | 44.59 ± 1.56 | 38.40 ± 1.91 | 38.17 ± 1.84 | 38.25 ± 1.64 | 122.537 | 0.000* | |
| Km(D) | 43.79 ± 1.56 | 37.85 ± 1.78 | 37.73 ± 1.70 | 37.77 ± 1.50 | 125.166 | 0.000* | |
| C.HOA(µm) | 0.38 ± 0.05 | 0.68 ± 0.10 | 0.70 ± 0.07 | 0.75 ± 0.07 | 34.059 | 0.000* | |
| C.Sph(µm) | 0.26 ± 0.07 | 0.52 ± 0.12 | 0.59 ± 0.10 | 0.59 ± 0.10 | 96.274 | 0.000* | |
| C.Coma(µm) | 0.23 ± 0.08 | 0.43 ± 0.10 | 0.43 ± 0.06 | 0.45 ± 0.11 | 28.341 | 0.000* | |
| C.Tre(µm) | 0.14 ± 0.05 | 0.22 ± 0.08 | 0.20 ± 0.08 | 0.19 ± 0.06 | 6.671 | 0.000* | |
| SR | 0 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 42.333 | 0.000* | |
*p < 0.05 was considered statistically significant. Values are presented as mean ± standard deviation. UDVA: uncorrected distance visual acuity; logMAR: logarithm of the minimum angle of resolution; SER: spherical equivalent; IOP: intraocular pressure; Q:anterior surface asphericity of the cornea; e: eccentricity; CCT: central corneal thickness; K1, K2, Km: corneal curvature; C.HOA: corneal higher-order aberrations at 6.0 mm pupil; C.Sph: corneal spherical aberration at 6.0 mm pupil; C.Coma: corneal coma aberration at 6.0 mm pupil; C.Tre: corneal trefoil aberration at 6.0 mm pupil; SR: strehl ratio.
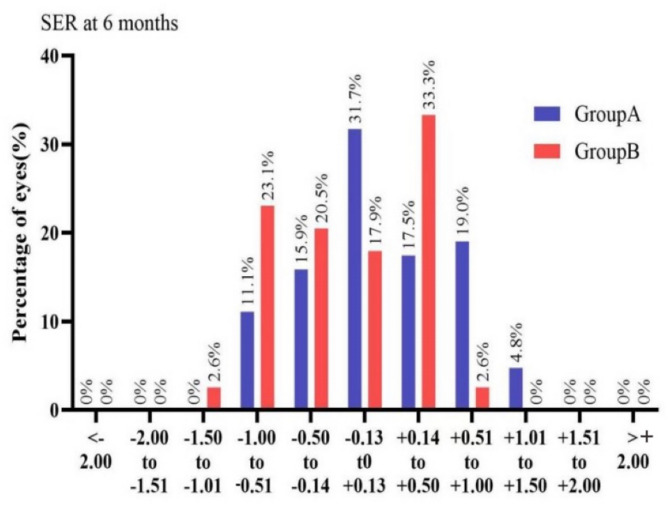
Meanwhile, Fig. 1 shows that at 6 months after surgery, 96.8% (in the low to moderate myopia group) and 92.3% (in the high myopia group) of the eyes in the two groups achieved UDVA of 20/20 (Snellen score) or above. Moreover, all patients achieved a CDVA of 20/20 (Snellen score). That is to say, no patient in either group lost one or more lines of CDVA.
Fig. 1.
Uncorrected distance visual acuity of both groups at 6 months after surgery. Group A represents the group with low to moderate myopia, while Group B represents the group with high myopia.
From Fig. 2, we can observe that in the low to moderate myopia group at 6 months after surgery, 65.1% of the eyes had SER within ± 0.5D, and 95.2% of the eyes had SER within ± 1.0D. In the high myopia group, 71.8% of the eyes had SER within ± 0.5D, and 97.4% of the eyes had SER within ± 1.0D.
Fig. 2.
The percentages of eyes with different spherical equivalent in both groups at 6 months after surgery. Group A represents the group with low to moderate myopia, while Group B represents the group with high myopia.
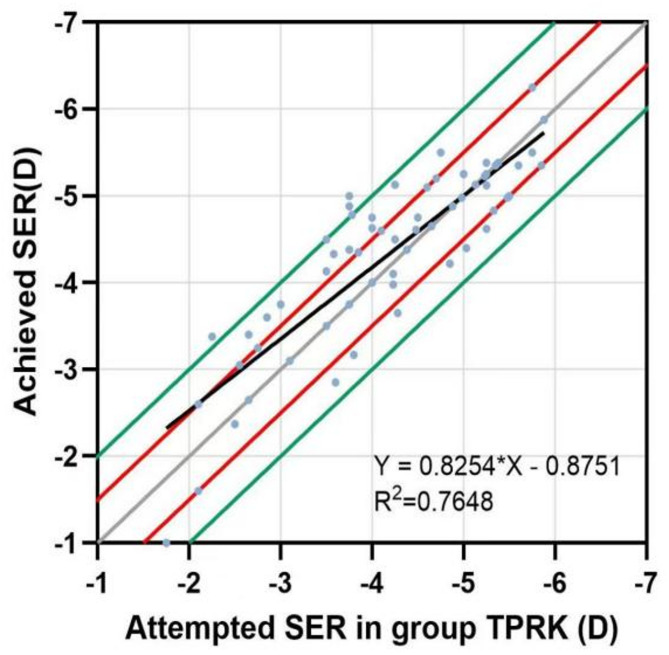
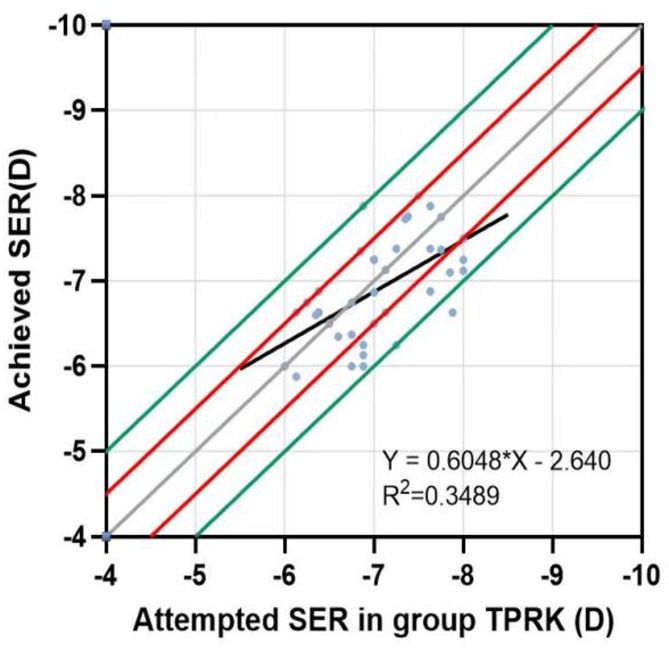
A linear regression was conducted for each group of attempts between the SER attempted and the SER achieved. The points above the regression line indicated overcorrection, with a certain degree of hyperopia remaining after surgery; those below the regression line indicated undercorrection, with a certain degree of myopia remaining. As shown in Figs. 3 and 4, both groups had similar convergence around the best-fit line. The slopes and coefficients (R2) of the low to moderate myopia group were 0.8254 and 0.7648, respectively, while those of the high myopia group were 0.6048 and 0.3489, respectively. This suggests that the scatter plot of the attempted and achieved SER in the low to moderate myopia group had better fitting predictability and accuracy.
Fig. 3.
Scatter plot of the SER attempted and achieved by the low-to-moderate myopia group at 6 months after surgery. The solid grey line represents the ideal result of y = x; the solid red line indicates that the difference between y and x is ± 0.5 D; the solid green line shows that the difference between y and x is ± 1.0 D.
Fig. 4.
Scatter plot of the SER attempted and achieved by the high myopia group at 6 months after surgery. The solid grey line represents the ideal result of y = x; the solid red line indicates that the difference between y and x is ± 0.5 D; the solid green line shows that the difference between y and x is ± 1.0 D.
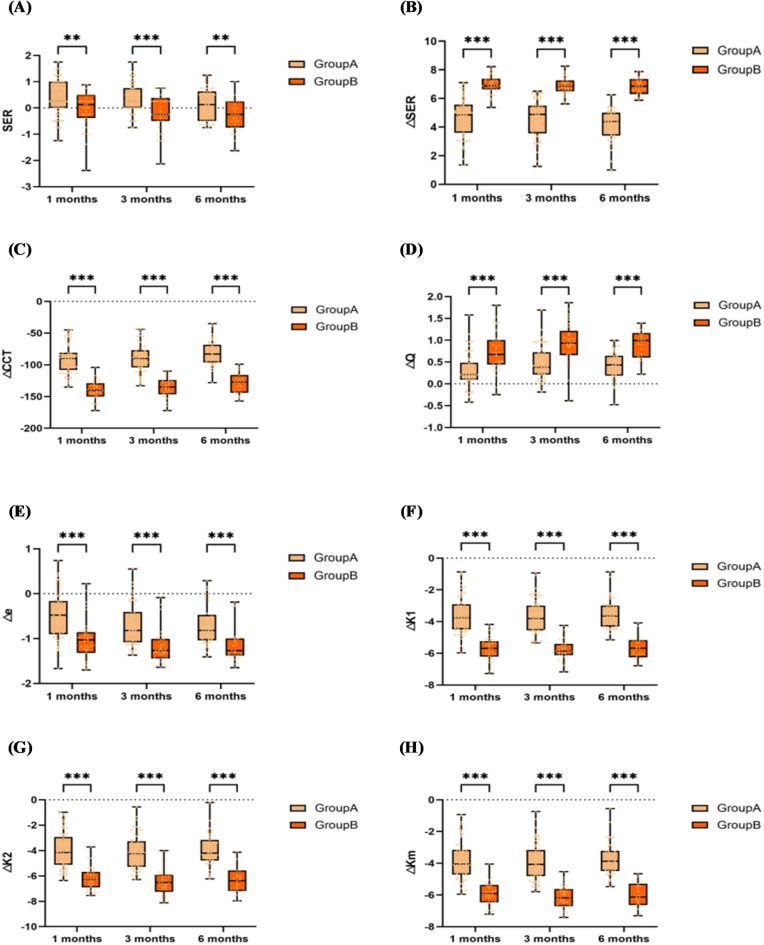
Comparison of changes at different time points after surgery among different groups
Comparison of inter-group variations in visual quality parameters
As shown in Fig. 5 (A), the difference in ΔC.HOA between groups at 1, 3, and 6 months postoperatively was significant (P < 0.001). The mean values of △C.HOA in the high myopia group at the same time points were higher than those in the low and moderate myopia group, and the mean values of the amount of change increased in both groups as time progressed.
Fig. 5.
Comparison of variations in visual quality parameters among groups(A) Comparison of △C.HOA at different postoperative time points(B) Comparison of △C.Coma at different postoperative time points(C) Comparison of △C.Sph at different postoperative time points(D) Comparison of △C.Tre at different postoperative time points(E) Comparison of △SR at different postoperative time points. Group A represents the group with low to moderate myopia, while Group B represents the group with high myopia.
As shown in Fig. 5 (B), the difference in △C.Coma between groups at 1, 3, and 6 months postoperatively was significant (P < 0.05). The mean values of △C.Coma in the high myopia group at the same time points were higher than those in the low and moderate myopia group, and the mean values of the change in both groups increased as time progressed.
As shown in Fig. 5 (C), ∆C.Sph differed significantly between groups at 1, 3, and 6 months postoperatively (P < 0.001)—the mean values of ΔC.Sph were higher in the high myopia group than in the low and moderate myopia group at the same time. The mean value of the amount of change in both groups at 3 months was greater than that at 1 month. At 6 months, the mean value of the change in the high myopia group decreased compared to that at 3 months. The mean value of the amount of change in the low and moderate myopia group was unchanged compared to that at 3 months, but the mean value of the change in the two groups was still greater than the mean value at 1 month at 6 months.
As shown in Fig. 5 (D), ∆C.Tre was not significantly different between groups at 1 (P = 0.62), 3 (P = 0.62), and 6 (P = 0.13) months postoperatively.
As shown in Fig. 5 (E), ∆SR did not differ significantly between groups at 1 (P = 0.59), 3 (P = 0.60), and 6 (P = 0.08) months postoperatively.
Comparison of inter-group variations in other refractive parameters
As shown in Fig. 6 (A), the SER was significantly different between groups at 1, 3, and 6 months postoperatively (P < 0.01). With the development of time, both groups showed a trend of increasing myopia after surgery. The mean SER values of both groups were positive at 1 month after surgery, and then gradually progressed towards negative values. At the same time, the mean SER values in the high myopia group all tended to be in the negative direction compared to the low and moderate myopia groups.
Fig. 6.
Comparison of Changes in Other Refractive Parameters among Groups(A) Comparison of SER at different postoperative time points(B) Comparison of △SER at different postoperative time points(C) Comparison of △CCT at different postoperative time points(D) Comparison of △Q at different postoperative time points(E) Comparison of △e at different postoperative time points(F) Comparison of △K1 at different postoperative time points(G) Comparison of △K2 at different postoperative time points(H) Comparison of △Km at different postoperative time points. Group A represents the group with low to moderate myopia, while Group B represents the group with high myopia.
As shown in Fig. 6 (B), △SER differed significantly between groups at 1, 3, and 6 months postoperatively (P < 0.001). The △SER of the high myopia group at the same time points were significantly higher than those of the low and moderate myopia group, and △the SER of both groups remained stable throughout the follow-up.
As shown in Fig. 6 (C), (D), (E), (F), (G), (H), ∆CCT, ∆Q, Δe, ΔK1, ΔK2, and ΔKm were significantly different between groups at 1, 3, and 6 months postoperatively (P < 0.001). Moreover, the mean values of the changes in the above parameters were higher in the high myopia group than in the low and moderate myopia group at the same time points.
Correlation analysis
Correlation between the variation values of visual quality parameters and spherical equivalent
Low to moderate myopia group:
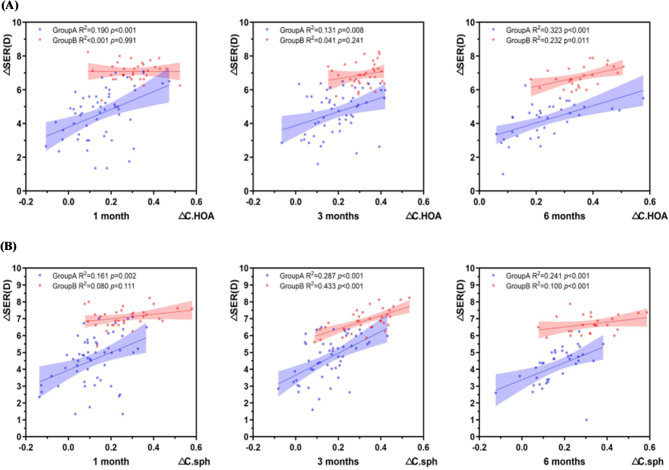
As shown in Fig. 7(A), ∆SER was positively correlated with ∆C.HOA (p < 0.01,r1 = 0.436; r3 = 0.362; r6 = 0.568);
Fig. 7.
Correlation between the variation values of visual quality parameters and spherical equivalent. (A) Correlation between △SER at different time points and △C.HOA (B) Correlation between △SER at different time points and △C.Sph. Group A represents the group with low to moderate myopia, while Group B represents the group with high myopia.
As shown in Fig. 7 (B), ∆SER was positively correlated with ∆C.Sph (P < 0.01,r1 = 0.401; r3 = 0.536; r6 = 0.491);
At 6 months after surgery, △SER was positively correlated with △C.Coma (P < 0.01, r6 = 0.422);
There was no correlation between ΔSER and ΔC.Tre, ΔSR (P > 0.05).
High myopia group:
As shown in Fig. 7(A), ∆SER and ∆C.HOA were positively correlated at 6 months (P = 0.011, r6 = 0.482);
As shown in Fig. 7 (B), ∆SER and ∆C.Sph were positively correlated at 3 and 6 months (P < 0.001, r3 = 0.658; r6 = 0.583); ΔSER and ΔSR were positively correlated at 3 and 6 months (P < 0.05, r3 = 0.430; r6 = 0.403);
There was no correlation between ΔSER and ΔC. Coma and ΔC.Tre (P > 0.05).
Correlation of values of changes in other refractive parameters with values of changes in refraction
Low to moderate myopia group:
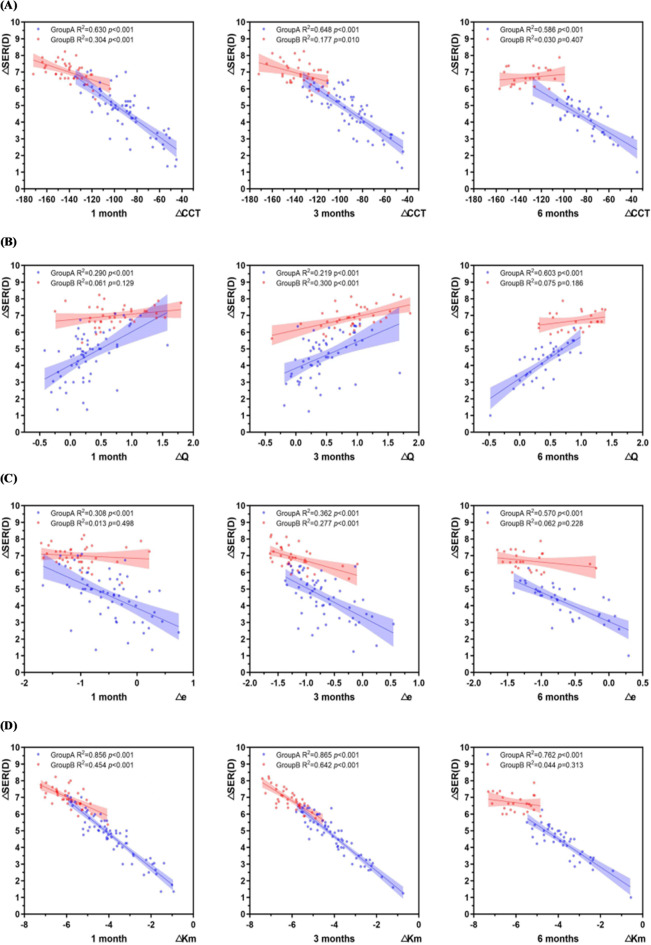
As shown in Fig. 8 (A), ΔSER was negatively correlated with ΔCCT (P < 0.001, r1 = −0.751; r3 = −0.805; r6 = −0.724);
Fig. 8.
Correlation between the variation values of other refractive parameters and spherical equivalent (A) Correlation between △SER and △CCT at different time points(B) Correlation between △SER and △Q at different time points(C) Correlation between △SER and △e at different time points(D) Correlation between △SER and △Km at different time points. Group A represents the group with low to moderate myopia, while Group B represents the group with high myopia.
As shown in Fig. 8 (B), ∆SER was positively correlated with ∆Q (P < 0.001, r1 = 0.553; r3 = 0.468; r6 = 0.73);
As shown in Fig. 8 (C), ∆SER was negatively correlated with ∆e (P < 0.001, r1 = −0.572; r3 = −0.602; r6 = −0.752);
As shown in Fig. 8 (D), ∆SER was negatively correlated with ∆Km (P < 0.001, r1 = −0.907; r3 = −0.930; r6 = −0.828).
High myopia group:
As shown in Fig. 8 (A), ΔSER and ΔCCT were negatively correlated at 1 and 3 months (P < 0.01,r1 = −0.552; r3 = −0.421);
As shown in Fig. 8 (B), ∆SER and ∆Q were positively correlated only at 3 months (p < 0.001,r3 = 0.548);
As shown in Fig. 8 (C), ∆SER and ∆e were negatively correlated only at 3 months (P < 0.001,r3=−0.526);
As shown in Fig. 8 (D), ΔSER and ΔKm were negatively correlated at 1 and 3 months (P < 0.001,r1 = −0.674; r3 = −0.801).
Astigmatism vector analysis
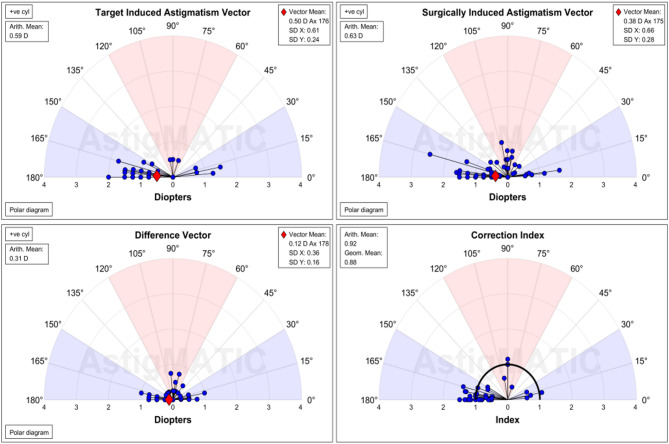
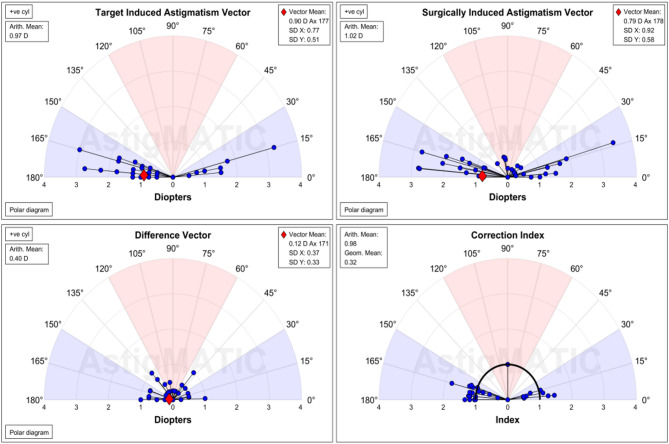
Using AstigMATIC software and the Alpins method, as shown in Figs. 9 and 10, the astigmatism was vectorially analysed and single-angle polar plots were constructed for both groups at 6 months postoperatively. Among them:
Fig. 9.
Vector analysis of astigmatism in the low to moderate myopia group.
Fig. 10.
Vector analysis of astigmatism in the high myopia group.
TIA: The expected corrected astigmatism, which is the vector difference between the preoperative astigmatism and the expected residual astigmatism that needs to be corrected by the surgery.
SIA: Surgical corrected astigmatism, which is the vector difference between the preoperative astigmatism and the residual astigmatism after the surgery.
DV: Error vector, the vector difference between TIA and SIA. When TIA is 0, DV is equal to the vector of the postoperative astigmatism.
CI: Correction ratio, the actual achieved part of the expected corrected astigmatism. When CI is 1, it indicates that the expected degree has been fully corrected; when CI is greater than 1, it indicates overcorrection; conversely, when CI is less than 1, it indicates undercorrection.
Although the high myopic group showed higher TIA and SIA, the two groups were similar in the DV and CI, with postoperative DV near 0 and CI near 1 in both groups, demonstrating the effectiveness of astigmatic correction.
Corneal haze
In this study involving patients with moderate to high myopia, cases of corneal epithelial-stromal opacity occurred in both groups at an early stage (within 1–2 months after surgery). It was all mild (barely visible with careful oblique illumination). By the time of the 3-month follow-up after surgery, the corneas of both groups were completely transparent. Regarding incidence, only seven eyes (11.1%) in the low to moderate to low myopia group showed slight opacity during the early follow-up period. Only six eyes (15.4%) showed slight opacity in the high myopia group during the early follow-up period. The two groups had no statistically significant difference (P = 0.53).
Discussion
In recent years, myopia has become one of the major problems affecting people’s eye health. With the development of science and technology, the safety and accuracy of corneal refractive surgery have been continuously improved, and more and more patients have favoured it. Among them, transPRK, with its advantages, has become one of the mainstream surgical methods for corneal refractive surgery. It has been shown that transPRK has demonstrated acceptable safety and efficacy in patients with low to moderate myopia and in patients with high myopia5,6,12,13,22,23, both of which have excellent visual outcomes, improved quality of life, and achieved high patient satisfaction24. It has also been shown25,26that transPRK is an effective and safe method of correcting low and moderate myopia. However, the stability and predictability of the correction may be reduced for high myopia, as it requires greater stromal ablation, which may lead to postoperative corneal instability.
Our study found that at 6 months after surgery, 96.8% (in the low and moderate myopia group) and 92.3% (in the high myopia group) of the eyes achieved UDVA of 20/20 or above. In the low and moderate myopia group at 6 months after surgery, 65.1% of the eyes had SER within ± 0.5D, and 95.2% had SER within ± 1.0D. In the high myopia group, 71.8% of the eyes had SER within ± 0.5D, and 97.4% had SER within ± 1.0D. Moreover, the mean values of △SER remained stable throughout the follow-up period. This is sufficient to prove the effectiveness of the surgery in terms of visual improvement and refractive correction.
A linear fit of the attempted SER to the realised SER for each group revealed that the fit was worse and slightly less predictable in the high myopia group than in the low to moderate myopia group, which may be because high preoperative refractive error affects the accuracy of the procedure to some extent. At 1 month postoperatively, the mean refractive error of both groups was still positive, and there was a tendency of refractive regression with the development of time, which was more evident in the high myopia group. This suggests that we pay attention to eye hygiene after surgery and correct the bad habits that may lead to myopia; otherwise, the myopia may increase again.It was found that no patient in either group lost one or more lines of CDVA, all patients were able to achieve a CDVA of 20/20 (Snellen’s score), and the central corneal thickness was greater than 360 microns at postoperative review in all patients, which can be used to demonstrate the safety of the procedure.
It is worth noting that we do not consider the current results to indicate that the percentages within ± 0.5D and ± 1.0D or the refractive accuracy in the high myopia group are better than those in the low to moderate myopia group. We are aware of the risk of myopic regression postoperatively. Since the corneal incision thickness is relatively lower in the low to moderate myopia group, we design postoperative residual hyperopia in some younger patients to offset the degree of refractive regression. When SER is between + 0.50 and + 1.50, the percentage in the low to moderate myopia group is significantly higher than in the high myopia group, which affects the statistical values of SER within the ± 0.50 and ± 1.0 ranges. In contrast, the percentage of SER within the − 0.50 to −1.50 range is higher in the high myopia group. From a long-term efficacy perspective, if refractive error changes at the same rate, when patients in the low to moderate myopia group with SER initially between + 0.50 and + 1.50 (23.8%) remain within the ± 1.0D range, patients in the high myopia group with SER initially between − 0.50 and − 1.50 (25.7%) were likely outside the ± 1.0D range. Therefore, from a long-term perspective, the high myopia group had poorer accuracy.
By comparing various parameters before and after the surgery, it was found that all parameters changed significantly after the surgery: UDVA (LogMAR), SER, IOP, CCT, K1, K2, and Km showed smaller values after the surgery, while C.HOA, C.Sph, C.Coma, C.Tre, and SR showed larger values after the surgery. Q changed from negative to positive, and e changed from positive to negative. Some studies suggest that the average intraocular pressure decreased after the surgery (compared with the baseline). This may be attributed to the relatively flat and thinner central cornea after transPRK and the absence of the anterior elastic layer27,28. Under physiological conditions, the anterior corneal surface gradually flattens from the center to the periphery, and the Q value is negative. After transPRK surgery, more central stromal tissue was ablated compared with the peripheral tissue, inevitably resulting in flattening of the anterior corneal surface, and the Q value changed in the direction of zero or even became positive16,29,30. Eccentricity is the degree of difference between the actual curvature of the cornea and the spherical surface curvature, and it also expresses the degree of peripheral flattening or steepening. The corneal morphology after transPRK became more flat, and the e value changed from positive to negative. It has been shown that the increase in Q value is significantly correlated with preoperative SER, ablation thickness, and ablation thickness/central corneal thickness16. This seems to explain the greater increase in surgical Q in the highly myopic group. Also, due to more central corneal cuts in the high myopia group, the difference between central and peripheral curvature of the cornea was greater, which may explain the greater eccentricity values in the high myopia group than in the low to moderate myopia group.
The therapeutic outcome of keratoconus surgery should not only satisfy the restoration of visual acuity but also improve visual quality. Regarding the problem of increased higher-order aberrations after surgery, a study31 suggested that the healing of corneal wounds after surgery and changes in the biomechanical properties of the cornea may be responsible for the increase in HOA. This study concluded that the increase in spherical aberration was mainly related to changes in corneal asphericity due to corneal epithelial healing and stromal fibrosis. Similarly, it has been suggested15that the changes of corneal HOA are mainly caused by the morphological changes of the cornea after surgery. Corneal asphericity is significantly altered after the surgery, the anterior corneal surface becomes flatter, and the light rays from the peripheral cornea focus earlier than those from the central cornea, thus showing an increase in the corneal aberration in the postoperative period. Roberts and Dupps32,33 suggested that central ablation surgery can reduce the tension of the residual peripheral lamellar segments, causing the peripheral force to pull the center outward, and the outward force will destroy the corneal morphology, which will further lead to changes in corneal distortion. It has also been previously shown34 that the magnitude of coma aberration is related to the degree of eccentricity. In this regard, we agree with the statement that there is a correlation between changes in corneal higher-order aberrations and changes in corneal morphology21,35and that more central corneal tissue than peripheral corneal tissue is cut during surgery, which increases postoperative spherical aberration. In addition, when the excimer laser cuts the peripheral cornea, the irradiation angle is inclined, and the laser irradiation energy density is reduced, which may increase coma aberration and affect image quality.
Regarding the comparison of HOA between high myopia and low to moderate myopia patients, Serrao36 pointed out that compared with low to moderate myopia patients treated with transPRK, the HOA in the eyes of high myopia patients increased more, which may be due to the difference in pupil diameter in high myopia affecting the HOA induced by transPRK37. It has been suggested15 that the higher the myopia, the deeper the corneal stroma that needs to be ablated, and the transition from the central corneal zone to the periphery of the cornea becomes steeper, which may lead to a significant change in Q value and thus increase corneal HOA, especially spherical aberration.
Our study found that the uncorrected distance visual acuity of the high myopia group at 6 months postoperatively was not low, and 84.6% of the patients’ subjective visual quality was not poor. No glare, halo, or night vision deterioration occurred. A related study38also concluded that patients with high myopia also have desirable visual quality, which may be related to the single-step platform of transPRK using the population-based corneal thickness curve to calculate the energy delivered to different parts of the cornea39,40. Studies41 suggest that the average change range observed in the HOA of patients with high myopia after transPRK is from 0.005 to 0.11 μm. It is expected that this range of change will not cause clinically significant visual function impairment.
When the astigmatism amplitude exceeds the physiological threshold, uncorrected astigmatism can lead to adult diplopia, glare, double vision, blurred vision and astigmatism. Considering that the study was conducted by converting astigmatism into spherical equivalent for statistics and conducting a grouping, this study also compared astigmatism correction using vector analysis. It was found that at 6 months, DV was around 0, and CI was around 1 in both groups, proving that the surgery was effective in correcting astigmatism.
The leading cause of corneal haze after surgery lies in the irregularity of the basement membrane and the active proliferation of the corneal epithelial cells. In severe cases, it may cause glare and misty vision, which may reduce the visual quality of patients. In this study, only 11.1% of the eyes in the low to moderate myopia group showed mild opacity in the early follow-up. In the high myopia group, only 15.4% of the eyes showed mild opacity in the early follow-up, and there was no statistically significant difference between the groups (P = 0.53). In this study, the incidence of haze was observed to be relatively low, which may be attributed to the use of SPT-assisted transPRK and cold saline irrigation after surgery. Compared with surface ablation without SPT, SPT-assisted transPRK resulted in a smoother corneal stroma bed, which was associated with faster corneal re-epithelialization, earlier postoperative vision recovery, shorter patient discomfort time, and less opacity occurrence42. Studies have shown that cold balanced saline solution surface ablation after PRK effectively prevents opacity43,44. At the same time, haze is also related to the patient’s care.
This study also has some limitations: (1) It is not a prospective randomized trial and has a relatively short follow-up period with an insufficiently large sample size. (2) Age and other possible influencing factors were not considered. (3)This study is limited to the evaluation of objective visual quality after surgery, and further combination with subjective visual quality is needed to evaluate the changes in postoperative visual quality more comprehensively.
Conclusion
TransPRK can safely and effectively correct the uncorrected distance visual acuity and refractive status of patients with low to moderate myopia, as well as those with high myopia. Compared with patients with low to moderate myopia, patients with high myopia show more growth in corneal higher-order aberrations, corneal spherical aberration, and corneal coma aberration at the same time point after surgery, and greater changes in corneal central thickness, Q value, e value, and corneal curvature. The change in SER is positively correlated with the change in corneal higher-order aberrations after surgery. Among the three aberrations, the change in SER is most strongly correlated with the change in corneal spherical aberration. It has a stronger correlation in the high myopia group at the same time point. The change in SER of the two groups of patients is positively correlated with the change in Q value in the early stage, and negatively correlated with the change in e value, corneal curvature, and corneal central thickness in the early stage.
Acknowledgements
We would like to acknowledge all the staff that participated in this study, and medical writers, proof-readers and editors.
Author contributions
ZS and QZ made substantial contributions to design of the work and revise the manuscript critically, drafted the manuscript, and agreed to be accountable for all aspects of the work. CL and MG contributed to data collection. SZ contributed to conduct the statistical analysis and draw diagrams. All authors have contributed to the final manuscript for important intellectual and read and approved the final manuscript.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability
The original data presented in the study is included in the article, further inquiries can be directed to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fricke, T. R. et al. Global prevalence of visual impairment associated with myopic macular degeneration and Temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br. J. Ophthalmol.102, 855–862 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan, I. G. et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res.62, 134–149 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Dong, L. et al. Prevalence and time trends of myopia in children and adolescents in China. Retina40, 399–411 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Flitcroft, D. I. et al. IMI - Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Invest Ophthalmol. Vis. Sci60, M20–M30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luger, M. H., Ewering, T. & Arba-Mosquera, S. Myopia correction with transepithelial photorefractive keratectomy versus femtosecond assisted laser in situ keratomileusis: one-year case-matched analysis. J. Cataract Refract. Surg.42 (7), 1579–1587 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Aslanides, I. M., Georgoudis, P. N., Selimis, V. D., Achyut, N. & Mukherjee Single-step transepithelial ASLA (SCHWIND) with mitomycin-C for correction of high myopia: long term follow-up. Clin. Ophtalmol. 9 (12), 33–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonios, R. et al. Single-step transepithelial versus alcohol-assisted photorefractive keratectomy in the treatment of high myopia: a comparative evaluation over 12 months. Br. J. Ophthalmol.101 (8), 1106–1112 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Wen, D. et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: A network Meta-analysis. Am. J. Ophthalmol.178 (6), 65–78 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Celik, U., Bozkurt, E., Celik, B., Ahmet Demirok, Omer, F. & Yilmaz Pain, wound healing and refractive comparison of mechanical and transepithelial debridement in photorefractive keratectomy for myopia: results of 1 year follow-up. Cont. Lens Anterior Eye. 37 (6), 420–426 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Chang, J. Y., Lin, P. Y., Hsu, C. C. & Catherine Jui-Ling Liu. Comparison of clinical outcomes of LASIK, Trans-PRK, and SMILE for correction of myopia. J Chin Med Assoc. 85 (2), 145–151 (2022). [DOI] [PubMed]
- 11.Gershoni, A. et al. Femtosecond laser assisted in situ keratomileusis (FS-LASIK) yields better results than transepithelial photorefractive keratectomy (Trans-PRK) for correction of low to moderate grade myopia. Eur. J. Ophthalmol.31, 2914–2922 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Guo, H., Hosseini-Moghaddam, S. M. & Hodge, W. Corneal Biomechanical properties after SMILE versus FLEX, LASIK, LASEK, or PRK: a systematic review and meta-analysis. BMC Ophthalmol.19, 167 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mounir, A. et al. Clinical outcomes of transepithelial photorefractive keratectomy versus femtosecond laser assisted keratomileusis for correction of high myopia in South Egyptian population. Int. J. Ophthalmol.13, 129–134 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang, J., Feng, Q., Ding, W., Peng, Y. & Long, K. Comparison of clinical results between trans-PRK and femtosecond LASIK for correction of high myopia. BMC Ophthalmol.20, 243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen, D. et al. Corneal surface ablation laser refractive surgery for the correction of myopia: a network meta-analysis. J. Refract. Surg.34, 726–735 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Wu, Y. et al. Corneal Asphericity and Higher-Order Aberrations after FS-LASIK and Trans-PRK for Myopia. J Ophthalmol. 2021, 3765046 (2021). [DOI] [PMC free article] [PubMed]
- 17.Zhang, Y. L., Xu, X. H., Cao, L. J. & Lei, L. Corneal curvature, asphericity, and aberrations after transepithelial photorefractive keratectomy and femtosecond laser-assisted in situ keratomileusis for myopia: a prospective comparative study. Indian J. Ophthalmol.68 (12), 2945–2949 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fini, M. E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res.18, 529–551 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Wilson, S. E., Liu, J. J. & Mohan, R. R. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res.18, 293–309 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Oliveira, C. M., Ferreira, A. & Franco, S. Wavefront analysis and Zernike polynomial decomposition for evaluation of corneal optical qualit. J. Cataract Refract. Surg.38 (2), 343–356 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Bottos, K. M. et al. Corneal asphericity and spherical aberration after refractive surgery. J. Cataract Refract. Surg.37 (6), 1109–1115 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Xi, L., Zhang, C. & He, Y. Single-step Transepithelial photorefractive keratectomy in the treatment of mild, moderate, and high myopia: six month results. BMC Ophthalmol. 18 (1), 209 (2018). [DOI] [PMC free article] [PubMed]
- 23.Ghadhfan, F., Al-Rajhi, A. & Wagoner, M. D. Laser in situ keratomileusis versus surface ablation: visual outcomes and complications. J. Cataract Refract. Surg.33, 2041–2048 (2007). [DOI] [PubMed] [Google Scholar]
- 24.SolomonKD et al. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology116, 691–701 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Shaher, A. et al. Comparison of clinical results between flap-on and flap-off techniques of epithelial-laser in situ keratomileusis in correction of low to moderate myopia in eyes with thin Corneas. Saudi J. Ophthalmol.27 (1), 31–35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirouzian, A., Thornton, J. A. & Ngo, S. A randomized prospective clinical trial comparing laser subepithelial keratomileusis and photorefractive keratectomy. Arch. Ophthalmol.122 (1), 11–16 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee, A., Shah, S., Bessant, D. A., Naroo, S. A. & Doyle, S. J. Reduction in intraocular pressure after excimer laser photorefractive keratectomy. Correlation Pretreatment Myopia Ophthalmology. 104, 355–359 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Javadi, M. A., Mirbabaei-Ghafghazi, F., Mirzade, M., Yazdani, S. & Yaseri, M. Steroid induced ocular hypertension following myopic photorefractive keratectomy. J. Ophthalmic Vis. Res.3, 42–46 (2008). [PMC free article] [PubMed] [Google Scholar]
- 29.Yu, M., Chen, M., Liu, W. & Dai, J. Comparative study of wave-front aberration and corneal asphericity after SMILE and LASEK for myopia: a short and long term study. BMC Ophthalmol.19 (1), 80 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, D. T. C., Holland, S. P., Verma, S., Hogden, J. & Arba-Mosquera, S. Postoperative corneal asphericity in low, moderate, and high myopic eyes after transepithelial PRK using a new pulse allocation. J. Refract. Surg.33 (12), 820–826 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Zheng, Z. et al. Comparison between aberration-free transepithelial photorefractive keratectomy and small incision lenticule extraction for correction of myopia and myopic astigmatism. Int. Ophthalmol.41 (1), 303–314 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Dupps, W. J. & Roberts, C. Effect of acute Biomechanical changes on corneal curvature after photokeratectomy. J. Refract. Surg.17 (6), 658–669 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Roberts, C. The cornea is not a piece of plastic. J. Refract. Surg.16 (4), 407–413 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Moreno-Barriuso, E. et al. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Invest. Ophthalmol. Vis. Sci.42 (6), 1396–1403 (2001). [PubMed] [Google Scholar]
- 35.Courtin, R., Saad, A., Grise-Dulac, A., Guilbert, E. & Gatinel, D. Changes to corneal aberrations and vision after monovision in patients with hyperopia after using a customized aspheric ablation profile to increase corneal asphericity (Q-factor). J. Refract. Surg.32 (11), 734–741 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Serrao, S., Lombardo, G., Ducoli, P. & Lombardo, M. Long-term corneal wavefront aberration variations after photorefractive keratectomy for myopia and myopic astigmatism. J. Cataract Refract. Surg.37 (9), 1655–1666 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Guneri Beser, B., Yildiz, E. & Turan Vural, E. Prognostic factors of visual quality after transepithelial photorefractive keratectomy in patients with low-to-moderate myopia. Indian J. Ophthalmol.68 (12), 2940–2944 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adib-Moghaddam, S. et al. Single-step transepithelial photorefractive keratectomy in high myopia: qualitative and quantitative visual functions. Int J. Ophthalmol10(3), 445–452 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adib-Moghaddam, S., Arba-Mosquera, S., Salmanian, B., Omidvari, A. H. & Noorizadeh, F. On-line pachymetry outcome of ablation in aberration free mode TransPRK. Eur. J. Ophthalmol.24 (4), 483–489 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Aslanides, I. M., Padroni, S., Arba Mosquera, S., Ioannides, A. & Mukherjee, A. Comparison of single-step reverse transepithelial all-surface laser ablation (ASLA) to alcohol-assisted photorefractive keratectomy. Clin. Ophthalmol.6, 973–980 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, J. et al. Effects of spherical aberration on visual acuity at different contrasts. J. Cataract Refract. Surg.35 (8), 1389–1395 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Aslanides, I. M. & Kymionis, G. D. Trans advanced surface laser ablation (TransPRK) outcomes using SmartPulse technology trans advanced surface laser ablation (TransPRK) outcomes using SmartPulseTech. Cont. Lens Anterior Eye. 40, 42–46 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Niizuma, T. et al. Cooling the cornea to prevent side effects of photorefractive keratectomy. J. Refract. Corneal Surg.10 (2 Suppl), S262–S266 (1994). [PubMed] [Google Scholar]
- 44.Kitazawa, Y. et al. Cooling effect on excimer laser photorefractive keratectomy. J. Cataract Refract. Surg.25 (10), 1349–1355 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data presented in the study is included in the article, further inquiries can be directed to the corresponding author.