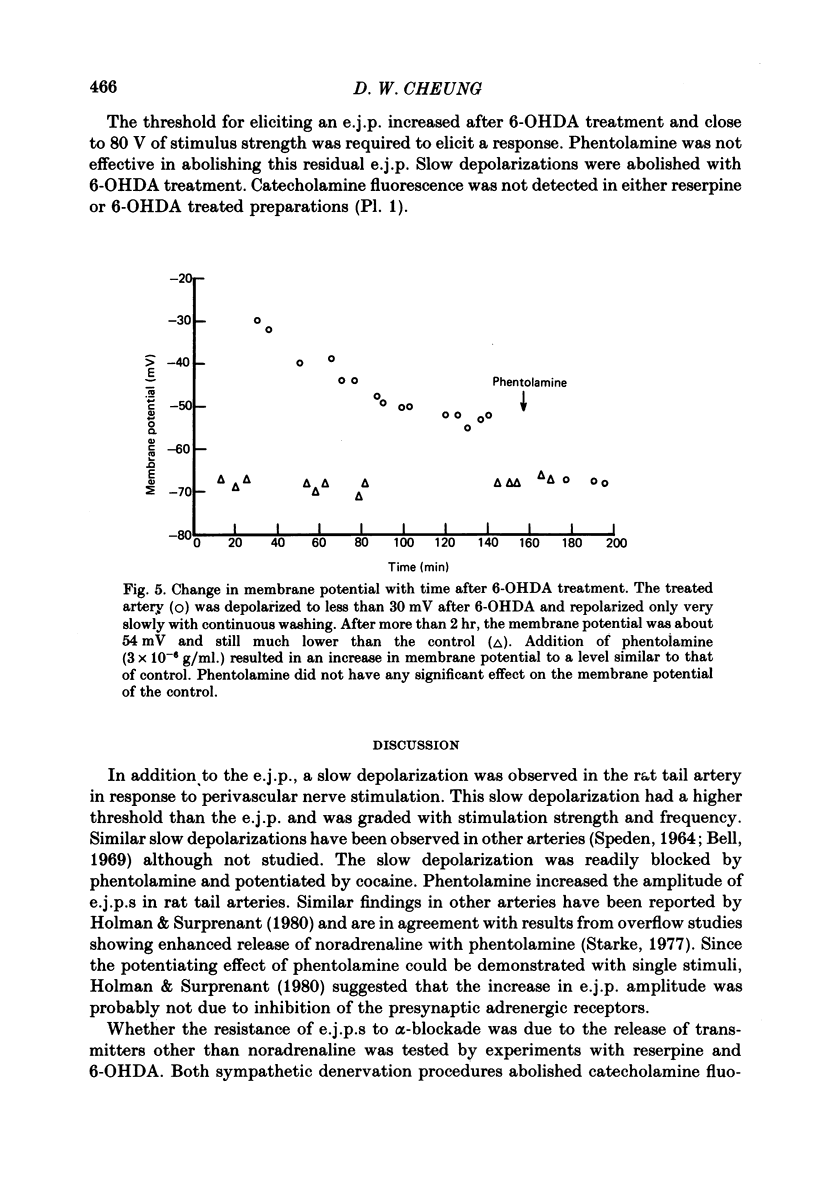
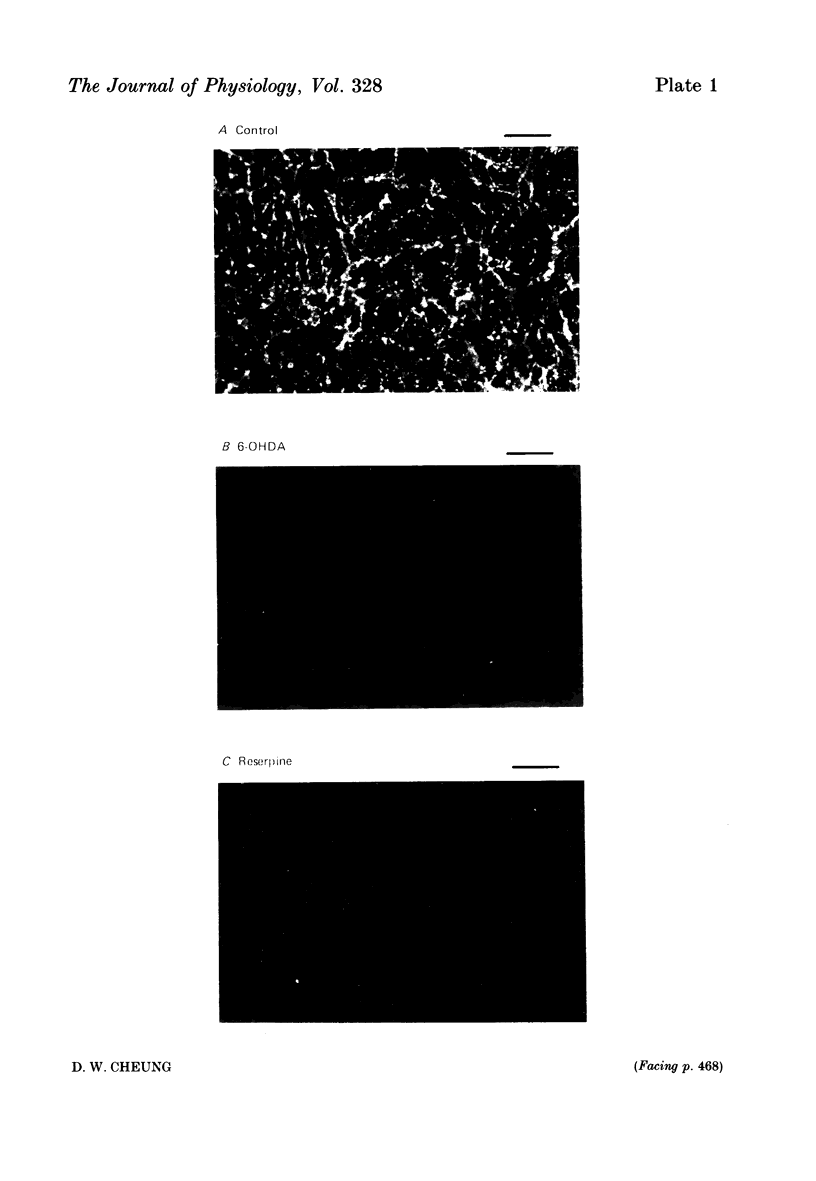
Abstract
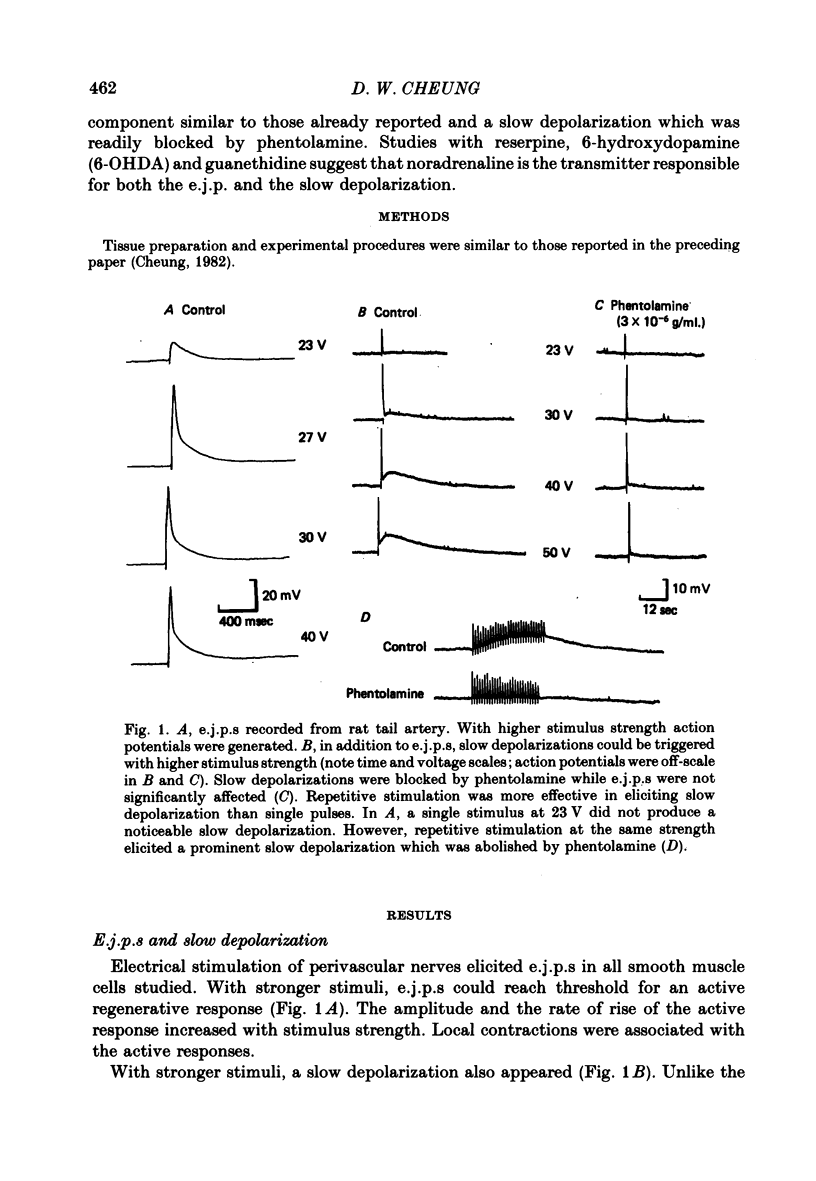
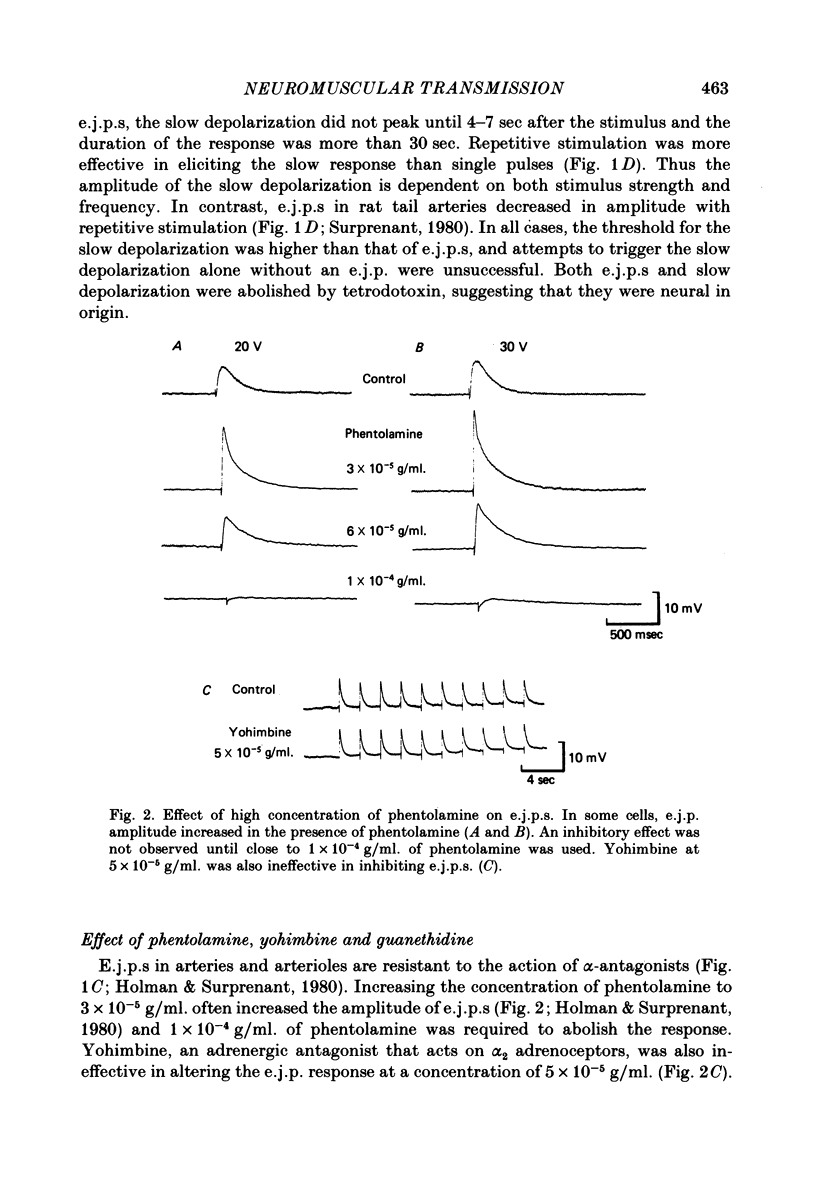
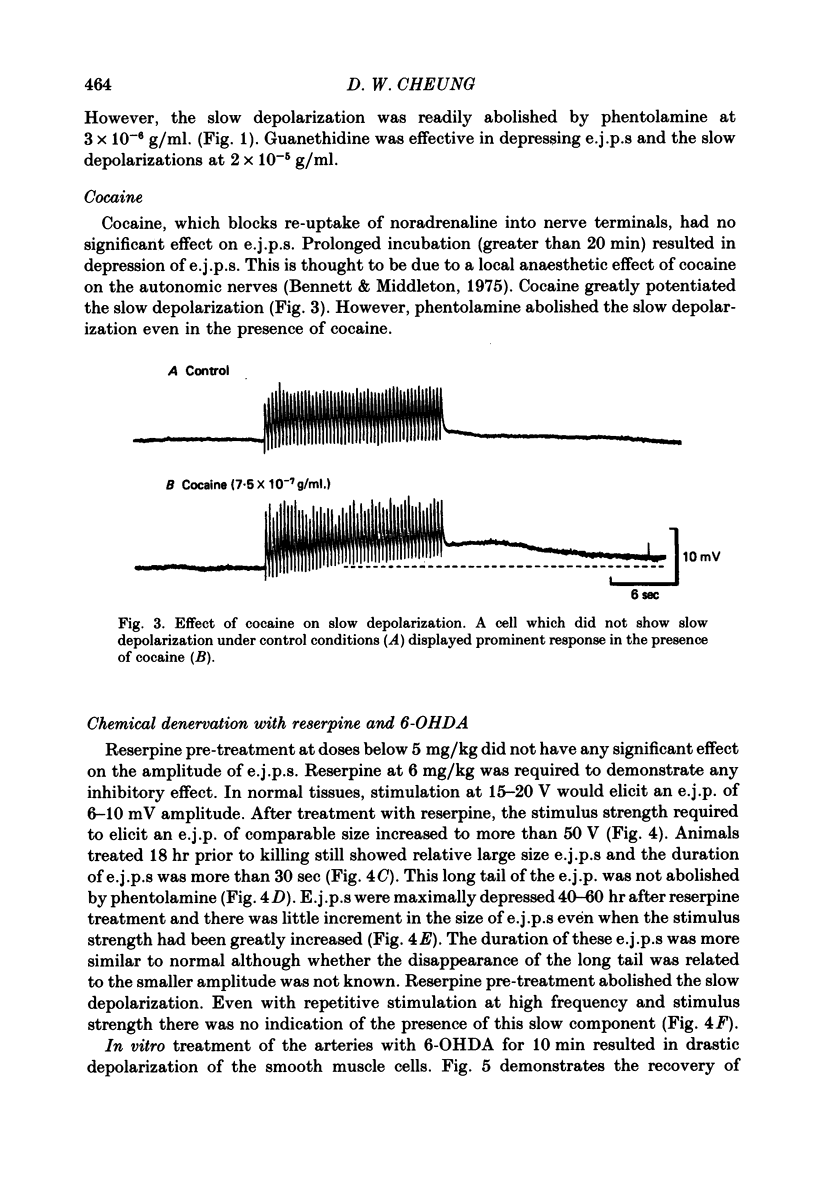
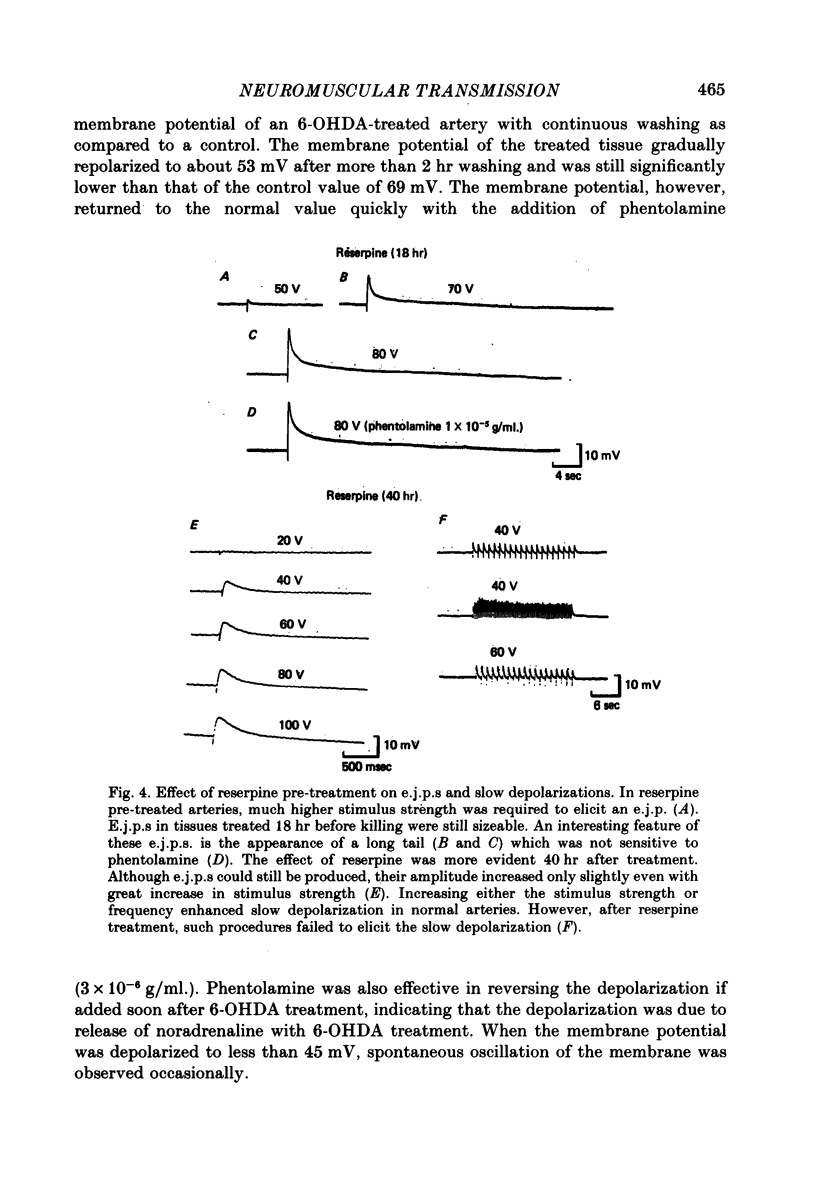
1. The response of rat tail arteries to stimulation of perivascular nerves was studied with intracellular micro-electrodes. 2. E.j.p.s were recorded from all smooth muscle cells. With higher stimulus strength, a slow depolarization that lasted for more than 30 sec also appeared. Repetitive stimulation was more effective in eliciting this slow component than were single pulses. 3. E.j.p.s were resistant to phentolamine and yohimbine. However, guanethidine and sympathetic denervation with reserpine and 6-hydroxydopamine depressed e.j.p.s. 4. The slow depolarization was readily blocked by phentolamine (1 x 10(-6) g/ml.) and potentiated by cocaine (1 x 10(-6) g/ml.). 5. It is proposed that vascular smooth muscle activity can be regulated neurally by both the e.j.p. and the slow depolarization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. Transmission from vasoconstrictor and vasodilator nerves to single smooth muscle cells of the guinea-pig uterine artery. J Physiol. 1969 Dec;205(3):695–708. doi: 10.1113/jphysiol.1969.sp008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Middleton J. An electrophysiological analysis of the effects of amine-uptake blockers and alpha-adrenoceptor blockers on adrenergic neuromuscular transmission. Br J Pharmacol. 1975 Sep;55(1):87–95. doi: 10.1111/j.1476-5381.1975.tb07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan J. A., Su C. Distribution theory of resistance of neurogenic vasoconstriction to alpha-receptor blockade in the rabbit. Circ Res. 1971 Feb;28(2):179–187. doi: 10.1161/01.res.28.2.179. [DOI] [PubMed] [Google Scholar]
- Hester R. K., Carrier O., Jr Tension development and associated calcium influx of control and reserpine pretreated rabbit aortae in response to norepinephrine, isoproterenol and acetylcholine. Arch Int Pharmacodyn Ther. 1978 May;233(1):21–41. [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiman M., Shibata S. Calcium influx and postjunctional supersensitivity in guinea pig aortic strips. Eur J Pharmacol. 1976 May;37(1):213–216. doi: 10.1016/0014-2999(76)90026-1. [DOI] [PubMed] [Google Scholar]
- McGrath J. C. Adrenergic and 'non-adrenergic' components in the contractile response of the vas deferens to a single indirect stimulus. J Physiol. 1978 Oct;283:23–39. doi: 10.1113/jphysiol.1978.sp012486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEDEN R. N. ELECTRICAL ACTIVITY OF SINGLE SMOOTH MUSCLE CELLS OF THE MESENTERIC ARTERY PRODUCED BY SPLANCHNIC NERVE STIMULATION IN THE GUINEA PIG. Nature. 1964 Apr 11;202:193–194. doi: 10.1038/202193a0. [DOI] [PubMed] [Google Scholar]
- Sedvall G., Thorson J. Adrenergic transmission at vasoconstrictor nerve terminals partially depleted of noradrenaline. Acta Physiol Scand. 1965 Jul;64(3):251–258. doi: 10.1111/j.1748-1716.1965.tb04175.x. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Surprenant A. A comparative study of neuromuscular transmission in several mammalian muscular arteries. Pflugers Arch. 1980 Jul;386(1):85–91. doi: 10.1007/BF00584192. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Vanhoutte P. M., Bohr D. F. Inactivation of released norepinephrine in rat tail artery by neuronal uptake. J Cardiovasc Pharmacol. 1980;2(2):121–132. doi: 10.1097/00005344-198003000-00004. [DOI] [PubMed] [Google Scholar]



