Abstract
1. Axonally transported organelles were detected optically in myelinated axons from Xenopus laevis at room temperature (21-23 °C). Details of the motion of organelles which were transported in the retrograde and anterograde directions were studied using filmed records.
2. A group of 133 organelles with a mean retrograde velocity of 0·91 μm/sec was compared with a group of thirty-nine organelles with a mean anterograde velocity of 0·93 μm/sec.
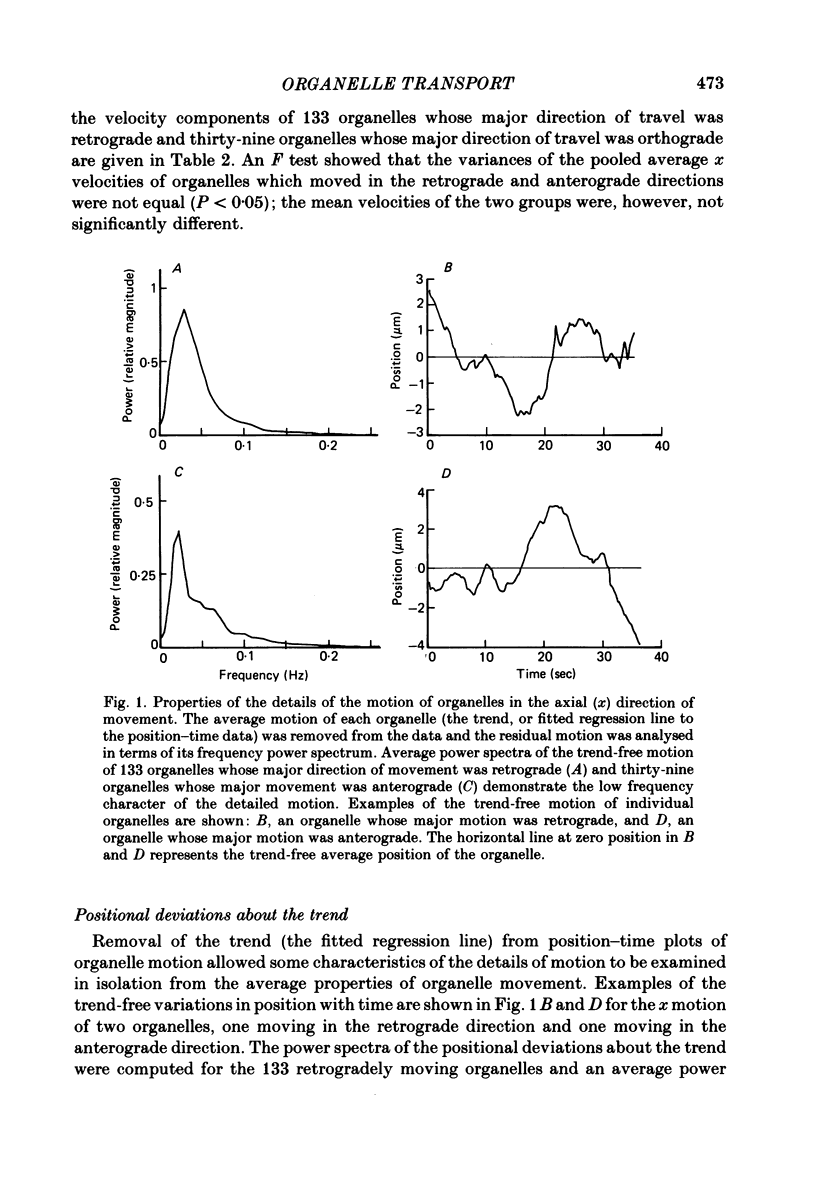
3. Averaged power spectra of the positional deviations about the mean positional change through time were constructed for organelles which travelled in the retrograde and anterograde directions. Most of the power in the two spectra was at frequencies below 0·2 Hz and each contained a single peak at 0·02-0·04 Hz. The power spectrum for retrograde organelle motion had a magnitude about twice that for anterograde organelle motion.
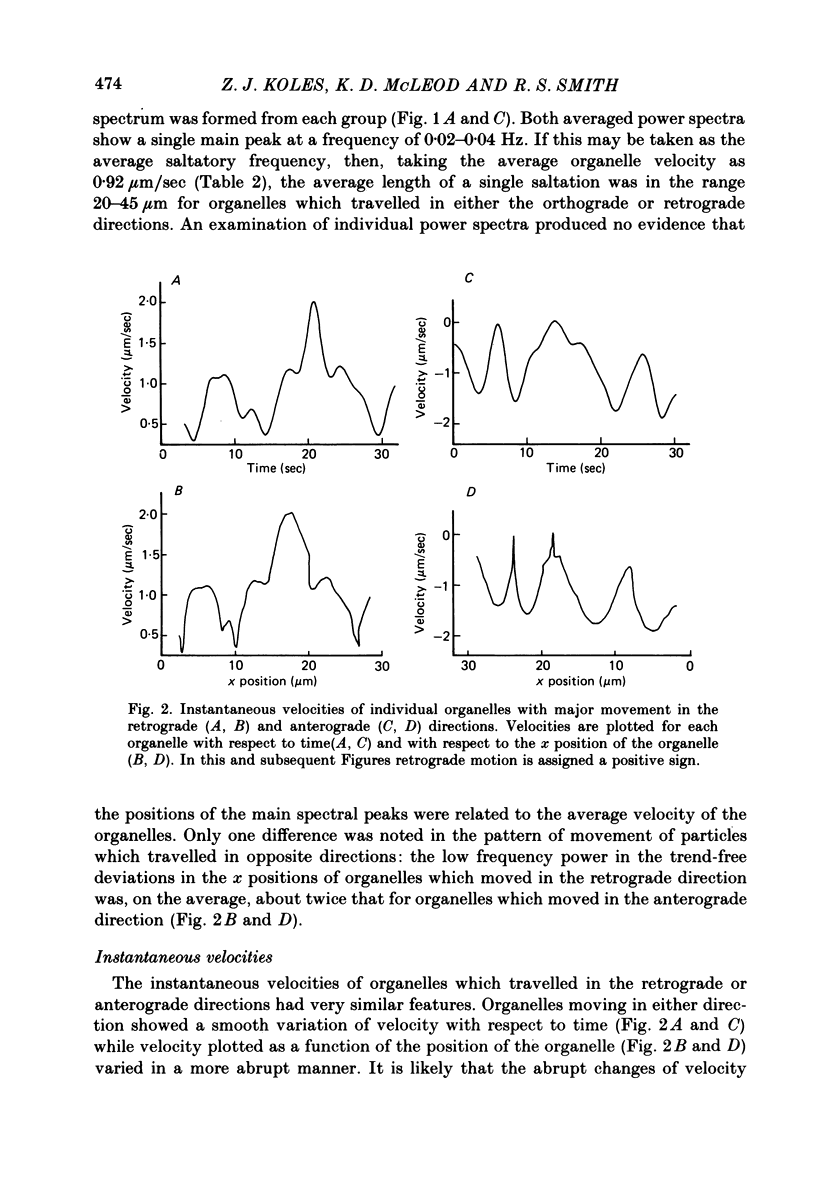
4. Estimates of the instantaneous velocity of organelles which travelled in either direction varied smoothly with time. Instantaneous velocity was not a smooth function of organelle position, (i.e. was `saltatory').
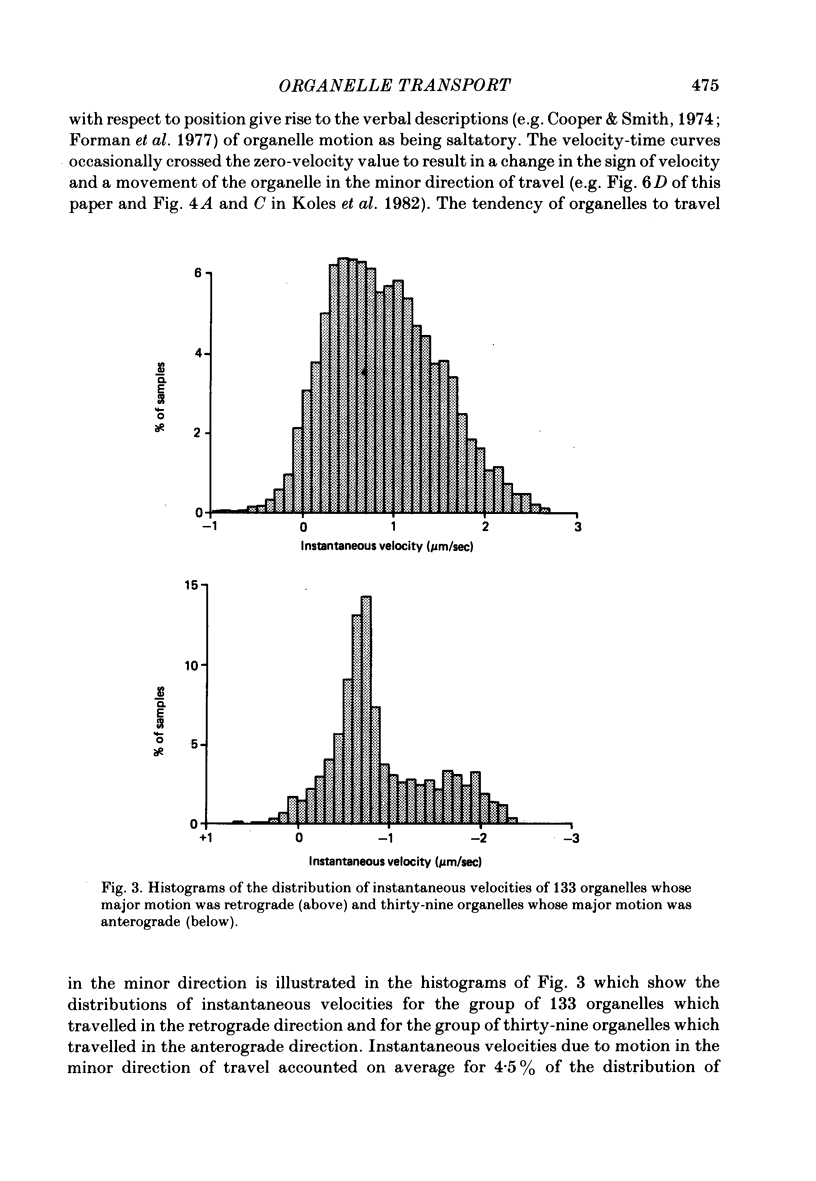
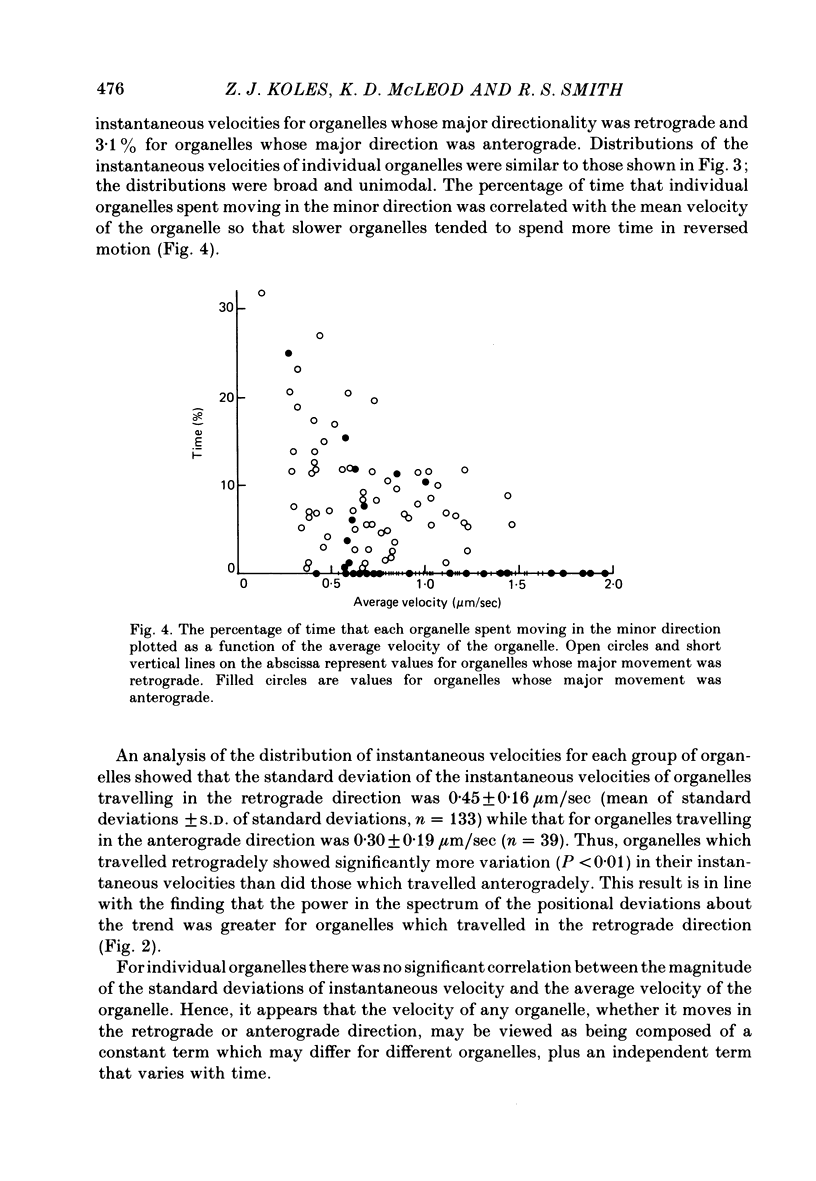
5. Histograms of the estimates for the groups of organelles whose major motion was retrograde or anterograde were broad, covering a range of about 3 μm/sec, were unimodal, and passed through zero to include a small group of values which indicated motion in the opposite (minor) direction.
6. Organelles spent, on average, more time moving in the minor direction the lower their mean velocity.
7. The variation in instantaneous velocity was greater for organelles which travelled in the retrograde direction than for those which travelled in the anterograde direction. No correlation was found between the variation of instantaneous velocity and the mean velocity of the organelles.
8. Images of organelles occasionally appeared to rotate while the organelle continued to move in the major direction of travel.
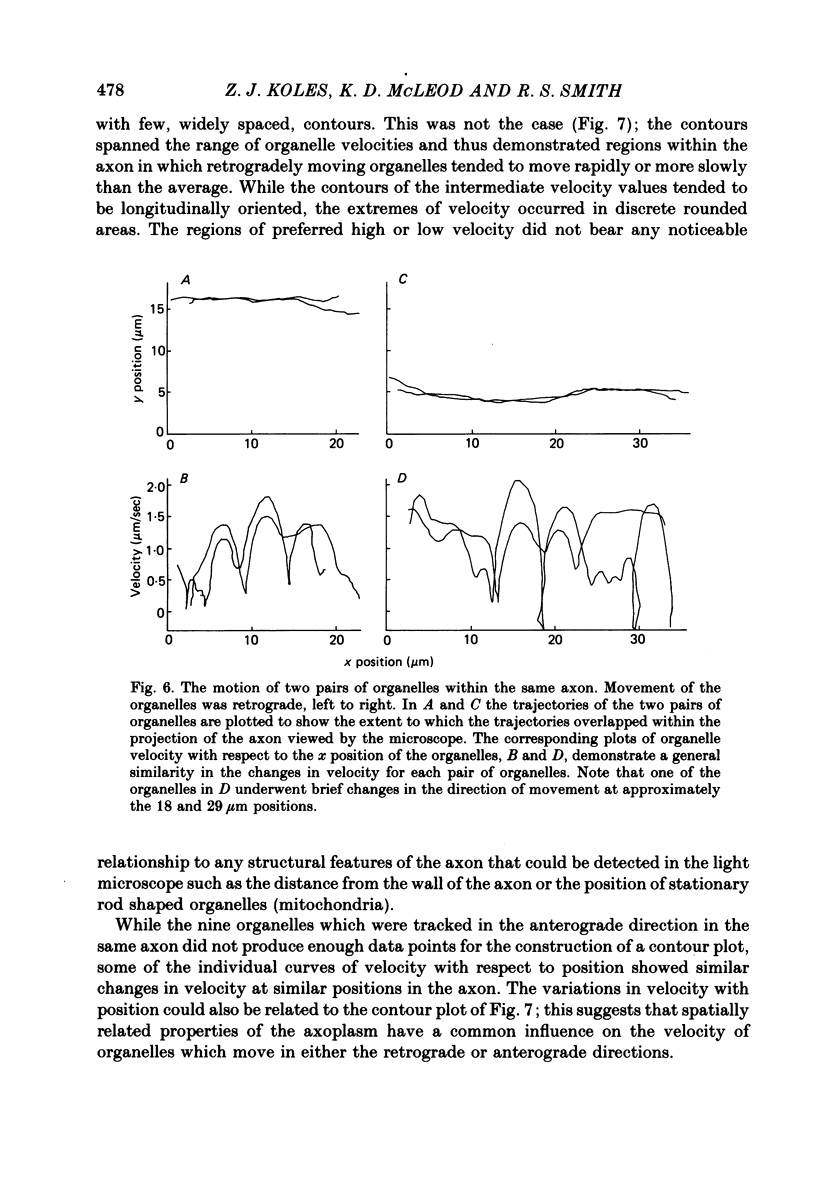
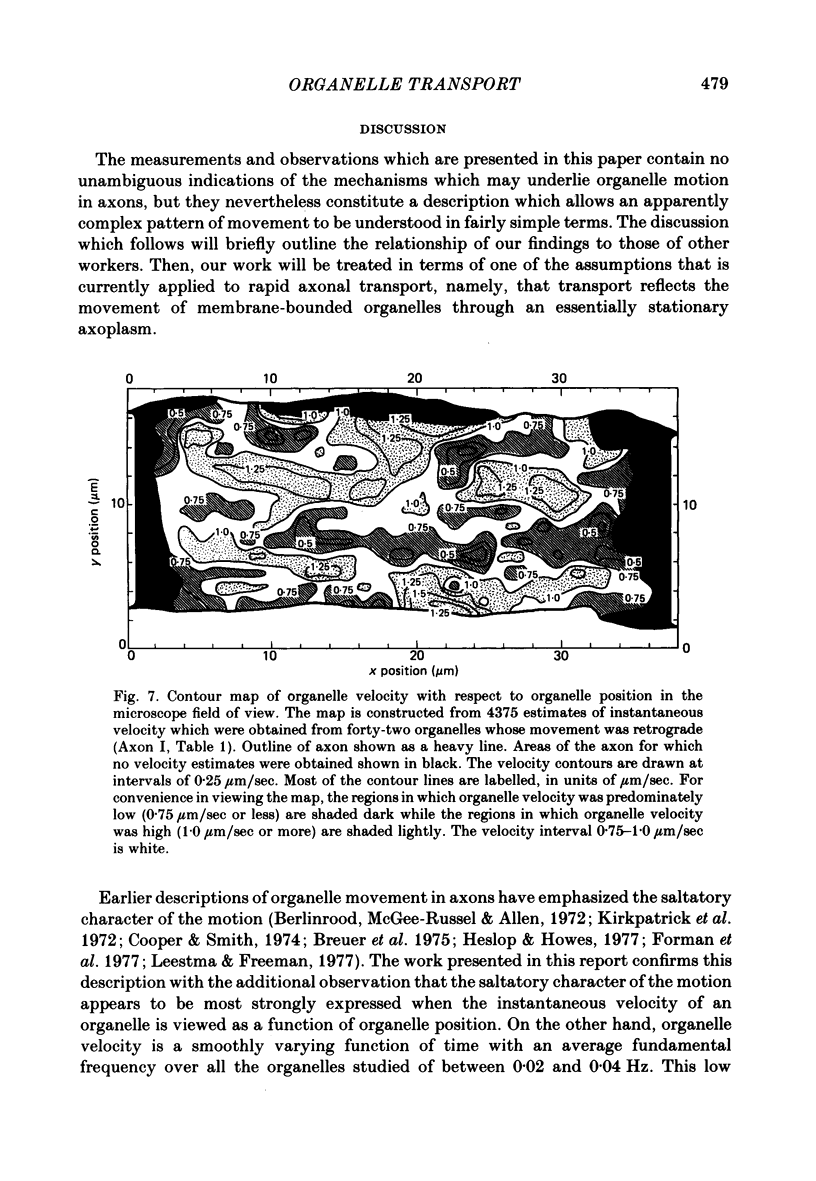
9. Evidence is presented that spatially related properties of the axon influence organelle velocity and that this influence is common to organelles which travel in the two major directions.
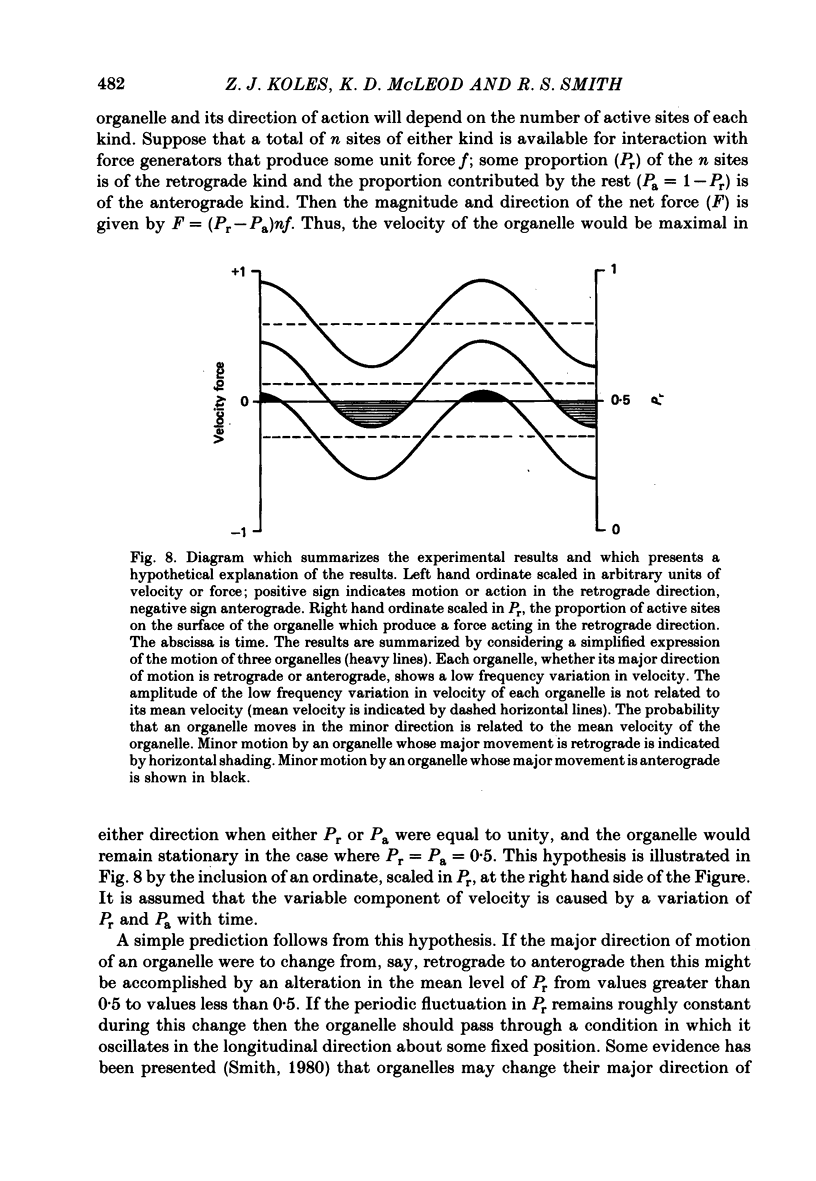
10. A hypothesis is presented to account for the findings. This supposes that each organelle travels through a stationary axoplasm and is propelled by the resultant of two opposing driving forces whose relative magnitude fluctuates with time. Spatially dependent properties of the axoplasm modify the postulated time-related cycle of motion.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Haga T., Kurokawa M. Retrograde axoplasmic transport: its continuation as anterograde transport. FEBS Lett. 1974 Oct 15;47(2):272–275. doi: 10.1016/0014-5793(74)81028-8. [DOI] [PubMed] [Google Scholar]
- Berlinrood M., McGee-Russell S. M., Allen R. D. Patterns of particle movement in nerve fibres in vitro. An analysis by photokymography and microscopy. J Cell Sci. 1972 Nov;11(3):875–886. doi: 10.1242/jcs.11.3.875. [DOI] [PubMed] [Google Scholar]
- Bisby M. A., Bulger V. T. Reversal of axonal transport at a nerve crush. J Neurochem. 1977 Aug;29(2):313–320. doi: 10.1111/j.1471-4159.1977.tb09624.x. [DOI] [PubMed] [Google Scholar]
- Bray J. J., Kon C. M., Breckenridge B. M. Reversed polarity of rapid axonal transport in chicken motoneurons. Brain Res. 1971 Oct 29;33(2):560–564. doi: 10.1016/0006-8993(71)90138-7. [DOI] [PubMed] [Google Scholar]
- Breuer A. C., Christian C. N., Henkart M., Nelson P. G. Computer analysis of organelle translocation in primary neuronal cultures and continuous cell lines. J Cell Biol. 1975 Jun;65(3):562–576. doi: 10.1083/jcb.65.3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. D., Smith R. S. The movement of optically detectable organelles in myelinated axons of Xenopus laevis. J Physiol. 1974 Oct;242(1):77–97. doi: 10.1113/jphysiol.1974.sp010695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman D. S., Padjen A. L., Siggins G. R. Axonal transport of organelles visualized by light microscopy: cinemicrographic and computer analysis. Brain Res. 1977 Nov 11;136(2):197–213. doi: 10.1016/0006-8993(77)90798-3. [DOI] [PubMed] [Google Scholar]
- Goldberg D. J., Goldman J. E., Schwartz J. H. Alterations in amounts and rates of serotonin transported in an axon of the giant cerebral neurone of Aplysia californica. J Physiol. 1976 Jul;259(2):473–490. doi: 10.1113/jphysiol.1976.sp011477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. J., Schwartz J. H., Sherbany A. A. Kinetic properties of normal and perturbed axonal transport of serotonin in a single identified axon. J Physiol. 1978 Aug;281:559–579. doi: 10.1113/jphysiol.1978.sp012439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein B., Forman D. S. Intracellular transport in neurons. Physiol Rev. 1980 Oct;60(4):1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- Gross G. W. The microstream concept of axoplasmic and dendritic transport. Adv Neurol. 1975;12:283–296. [PubMed] [Google Scholar]
- Hammond G. R., Smith R. S. Inhibition of the rapid movement of optically detectable axonal particles colchicine and vinblastine. Brain Res. 1977 Jun 10;128(2):227–242. doi: 10.1016/0006-8993(77)90990-8. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick J. B., Bray J. J., Palmer S. M. Visualization of axoplasmic flow in vitro by Nomarski microscopy. Comparison to rapid flow of radioactive proteins. Brain Res. 1972 Aug 11;43(1):1–10. doi: 10.1016/0006-8993(72)90270-3. [DOI] [PubMed] [Google Scholar]
- Leestma J. E., Freeman S. S. Computer-assisted analysis of particulate axoplasmic flow in organized CNS tissue cultures. J Neurobiol. 1977 Sep;8(5):453–467. doi: 10.1002/neu.480080506. [DOI] [PubMed] [Google Scholar]
- Lubińska L., Niemierko S. Velocity and intensity of bidirectional migration of acetylcholinesterase in transected nerves. Brain Res. 1971 Apr 2;27(2):329–342. doi: 10.1016/0006-8993(71)90258-7. [DOI] [PubMed] [Google Scholar]
- Ochs S., Worth R. M., Chan S. Y. Calcium requirement for axoplasmic transport in mammalian nerve. Nature. 1977 Dec 22;270(5639):748–750. doi: 10.1038/270748a0. [DOI] [PubMed] [Google Scholar]
- Rubinow S. I., Blum J. J. A theoretical approach to the analysis of axonal transport. Biophys J. 1980 Apr;30(1):137–147. doi: 10.1016/S0006-3495(80)85082-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt F. O. Fibrous proteins--neuronal organelles. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1092–1101. doi: 10.1073/pnas.60.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. S., McLeod K. D. Unusual particle trajectories and structural arrangements in myelinated nerve fibers. Can J Physiol Pharmacol. 1979 Oct;57(10):1182–1186. doi: 10.1139/y79-176. [DOI] [PubMed] [Google Scholar]
- Smith R. S. The short term accumulation of axonally transported organelles in the region of localized lesions of single myelinated axons. J Neurocytol. 1980 Feb;9(1):39–65. doi: 10.1007/BF01205226. [DOI] [PubMed] [Google Scholar]


