Abstract
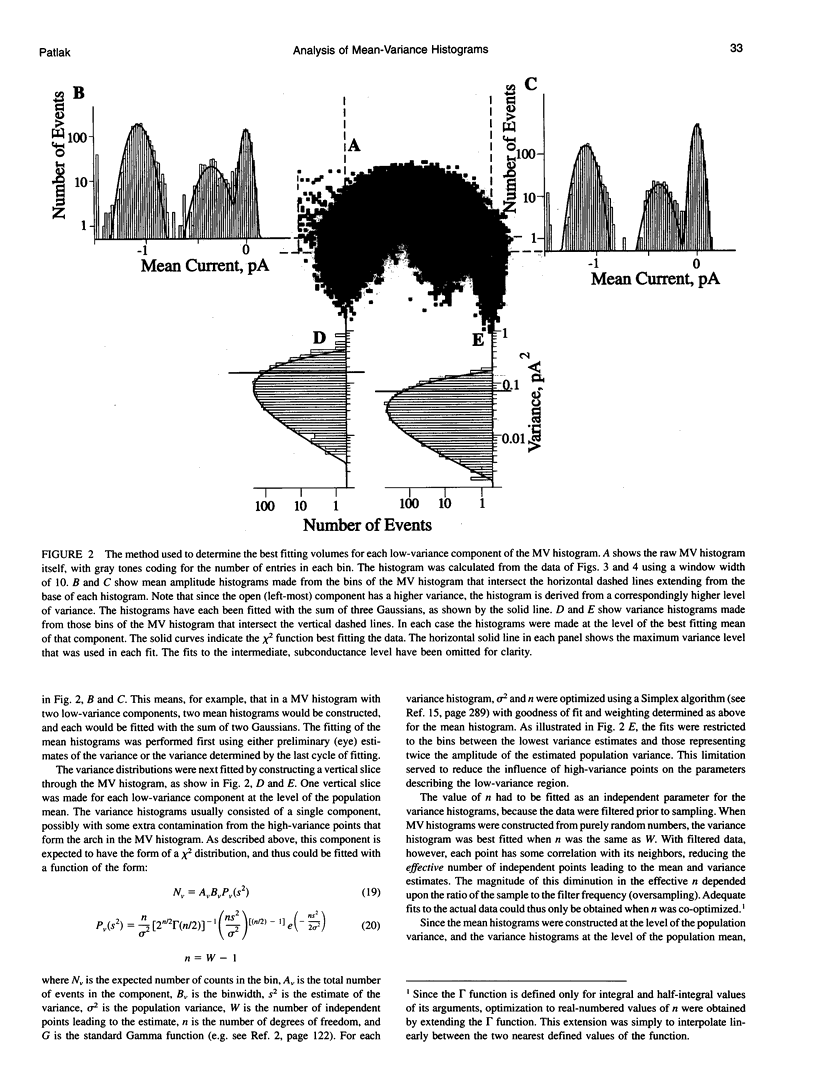
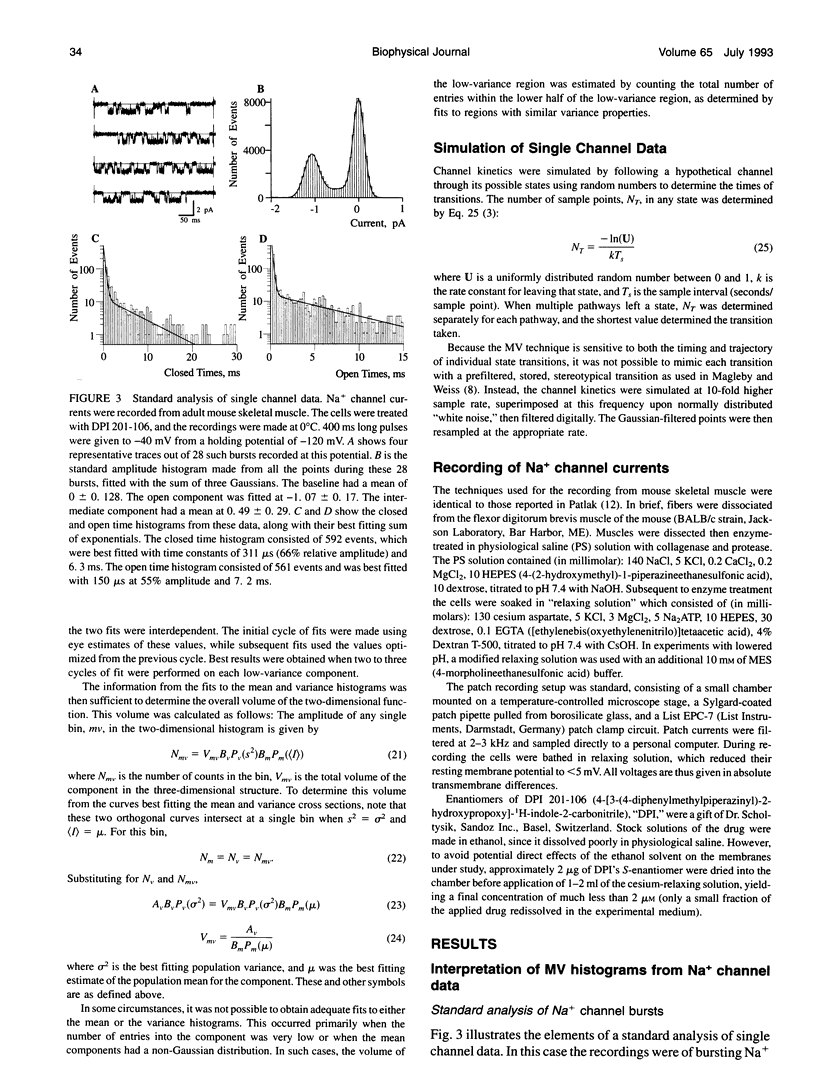
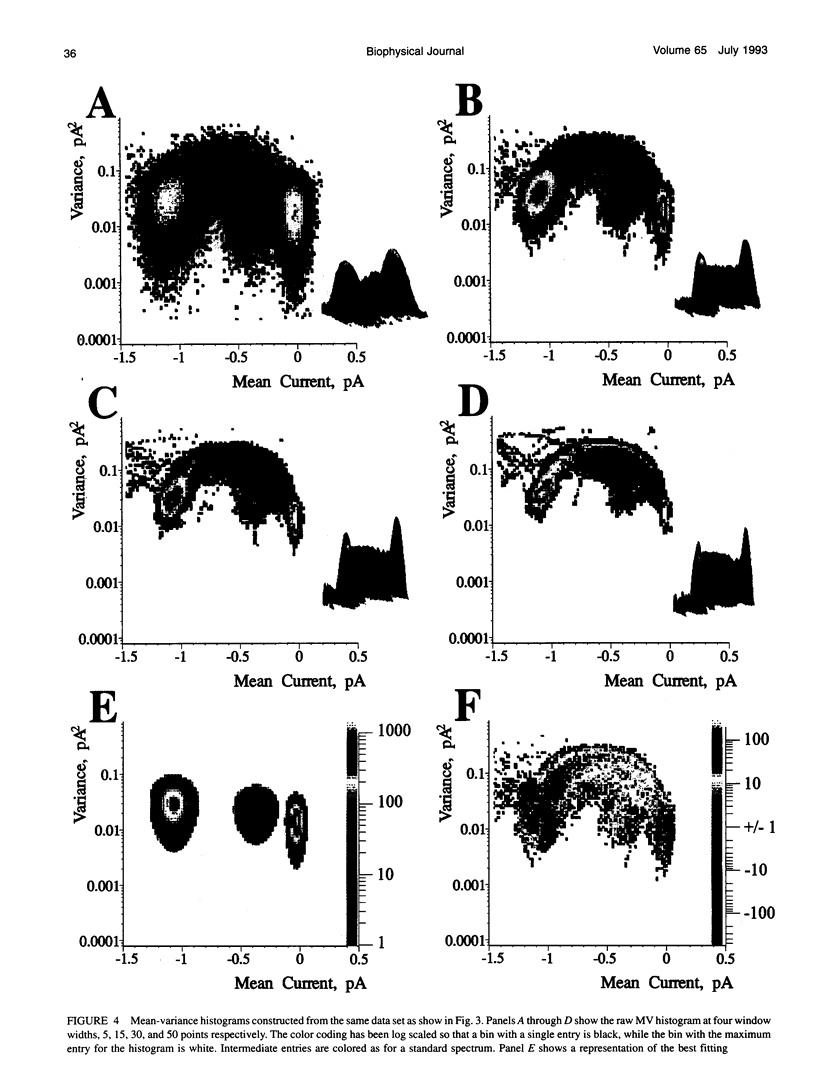
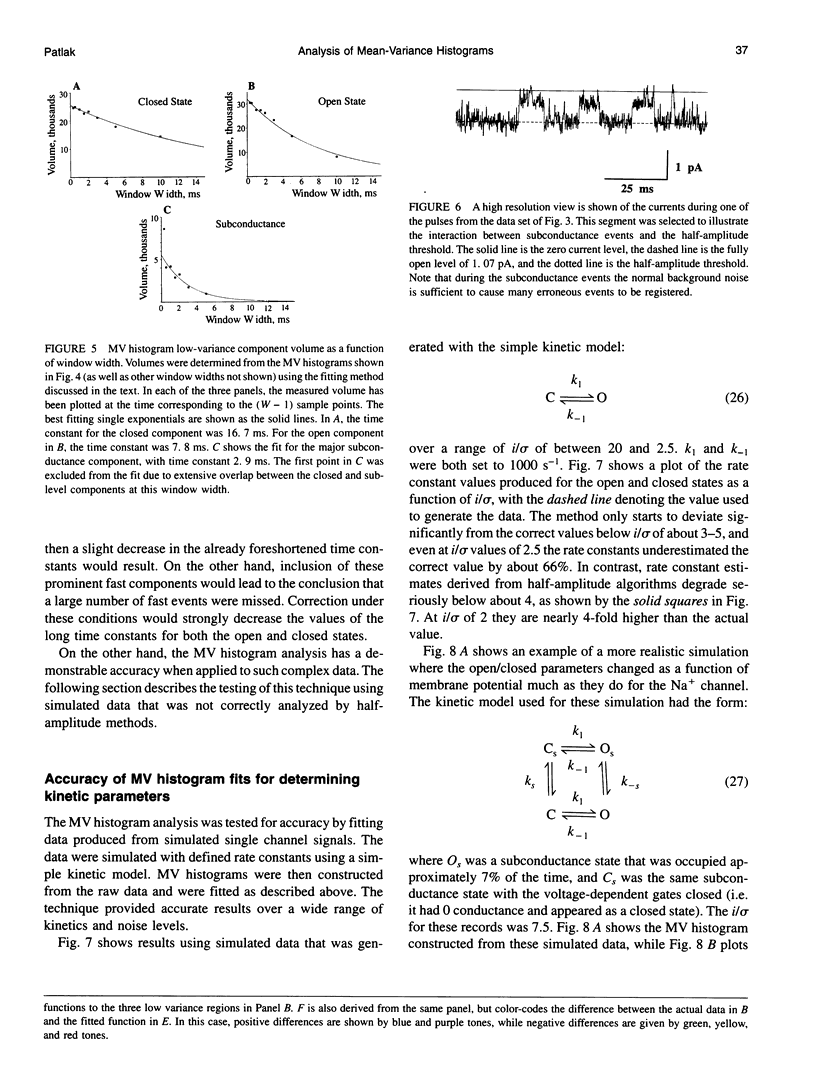
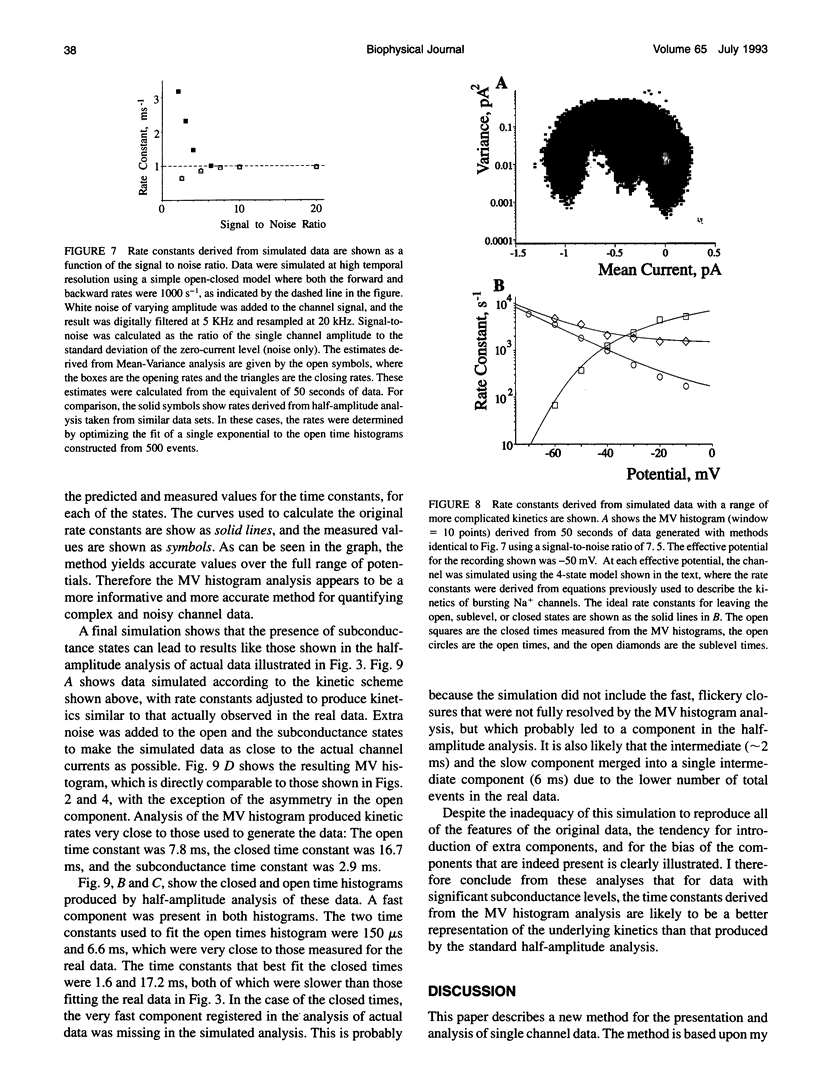
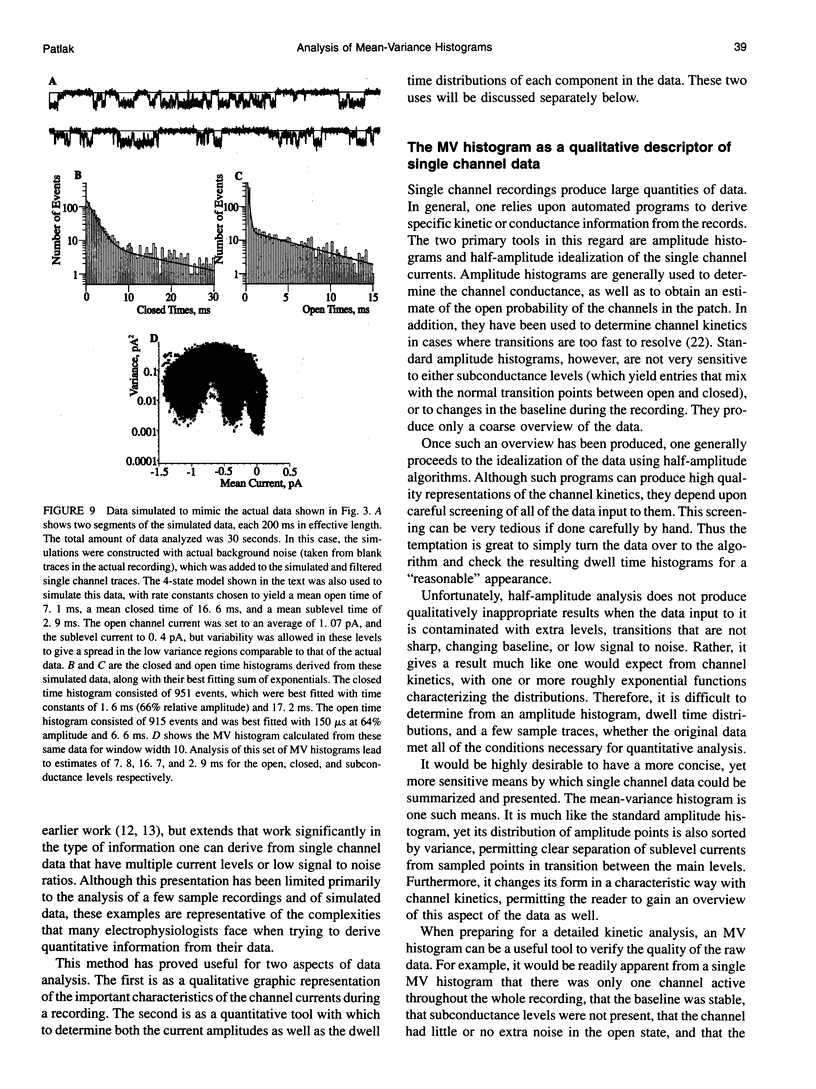
The measurement of single ion channel kinetics is difficult when those channels exhibit subconductance events. When the kinetics are fast, and when the current magnitudes are small, as is the case for Na+, Ca2+, and some K+ channels, these difficulties can lead to serious errors in the estimation of channel kinetics. I present here a method, based on the construction and analysis of mean-variance histograms, that can overcome these problems. A mean-variance histogram is constructed by calculating the mean current and the current variance within a brief "window" (a set of N consecutive data samples) superimposed on the digitized raw channel data. Systematic movement of this window over the data produces large numbers of mean-variance pairs which can be assembled into a two-dimensional histogram. Defined current levels (open, closed, or sublevel) appear in such plots as low variance regions. The total number of events in such low variance regions is estimated by curve fitting and plotted as a function of window width. This function decreases with the same time constants as the original dwell time probability distribution for each of the regions. The method can therefore be used: 1) to present a qualitative summary of the single channel data from which the signal-to-noise ratio, open channel noise, steadiness of the baseline, and number of conductance levels can be quickly determined; 2) to quantify the dwell time distribution in each of the levels exhibited. In this paper I present the analysis of a Na+ channel recording that had a number of complexities. The signal-to-noise ratio was only about 8 for the main open state, open channel noise, and fast flickers to other states were present, as were a substantial number of subconductance states. "Standard" half-amplitude threshold analysis of these data produce open and closed time histograms that were well fitted by the sum of two exponentials, but with apparently erroneous time constants, whereas the mean-variance histogram technique provided a more credible analysis of the open, closed, and subconductance times for the patch. I also show that the method produces accurate results on simulated data in a wide variety of conditions, whereas the half-amplitude method, when applied to complex simulated data shows the same errors as were apparent in the real data. The utility and the limitations of this new method are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. H., Krishnamurthy V., Moore J. B. Adaptive processing techniques based on hidden Markov models for characterizing very small channel currents buried in noise and deterministic interferences. Philos Trans R Soc Lond B Biol Sci. 1991 Dec 30;334(1271):357–384. doi: 10.1098/rstb.1991.0122. [DOI] [PubMed] [Google Scholar]
- Chung S. H., Moore J. B., Xia L. G., Premkumar L. S., Gage P. W. Characterization of single channel currents using digital signal processing techniques based on Hidden Markov Models. Philos Trans R Soc Lond B Biol Sci. 1990 Sep 29;329(1254):265–285. doi: 10.1098/rstb.1990.0170. [DOI] [PubMed] [Google Scholar]
- Gollasch M., Hescheler J., Quayle J. M., Patlak J. B., Nelson M. T. Single calcium channel currents of arterial smooth muscle at physiological calcium concentrations. Am J Physiol. 1992 Nov;263(5 Pt 1):C948–C952. doi: 10.1152/ajpcell.1992.263.5.C948. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Weiss D. S. Estimating kinetic parameters for single channels with simulation. A general method that resolves the missed event problem and accounts for noise. Biophys J. 1990 Dec;58(6):1411–1426. doi: 10.1016/S0006-3495(90)82487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Weiss D. S. Identifying kinetic gating mechanisms for ion channels by using two-dimensional distributions of simulated dwell times. Proc Biol Sci. 1990 Sep 22;241(1302):220–228. doi: 10.1098/rspb.1990.0089. [DOI] [PubMed] [Google Scholar]
- Nagy K. Subconductance states of single sodium channels modified by chloramine-T and sea anemone toxin in neuroblastoma cells. Eur Biophys J. 1987;15(3):129–132. doi: 10.1007/BF00263676. [DOI] [PubMed] [Google Scholar]
- Nilius B., Vereecke J., Carmeliet E. Different conductance states of the bursting Na channel in guinea-pig ventricular myocytes. Pflugers Arch. 1989 Jan;413(3):242–248. doi: 10.1007/BF00583536. [DOI] [PubMed] [Google Scholar]
- Patlak J. B. Sodium channel subconductance levels measured with a new variance-mean analysis. J Gen Physiol. 1988 Oct;92(4):413–430. doi: 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio-Pinto E., Thornhill W. B., Duch D. S., Levinson S. R., Urban B. W. Neuraminidase treatment modifies the function of electroplax sodium channels in planar lipid bilayers. Neuron. 1990 Nov;5(5):675–684. doi: 10.1016/0896-6273(90)90221-z. [DOI] [PubMed] [Google Scholar]
- Schreibmayer W., Tritthart H. A., Schindler H. The cardiac sodium channel shows a regular substate pattern indicating synchronized activity of several ion pathways instead of one. Biochim Biophys Acta. 1989 Nov 17;986(1):172–186. doi: 10.1016/0005-2736(89)90288-5. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]