Abstract
Although knowledge of the pKa values and charge states of individual residues is critical to understanding the role of electrostatic effects in protein structure and function, calculating these quantities is challenging because of the sensitivity of these parameters to the position and distribution of charges. Values for many different proteins which agree well with experimental results have been obtained with modified Tanford-Kirkwood theory in which the protein is modeled as a sphere (reviewed in Ref. 1); however, convergence is more difficult to achieve with finite difference methods, in which the protein is mapped onto a grid and derivatives of the potential function are calculated as differences between the values of the function at grid points (reviewed in Ref. 6). Multigrid methods, in which the size of the grid is varied from fine to coarse in several cycles, decrease computational time, increase rates of convergence, and improve agreement with experiment. Both the accuracy and computational advantage of the multigrid approach increase with grid size, because the time required to achieve a solution increases slowly with grid size. We have implemented a multigrid procedure for solving the nonlinear Poisson-Boltzmann equation, and, using lysozyme as a test case, compared calculations for several crystal forms, different refinement procedures, and different charge assignment schemes. The root mean square difference between calculated and experimental pKa values for the crystal structure which yields best agreement with experiment (1LZT) is 1.1 pH units, with the differences in calculated and experimental pK values being less than 0.6 pH units for 16 out of 21 residues. The calculated titration curves of several residues are biphasic.
Full text
PDF

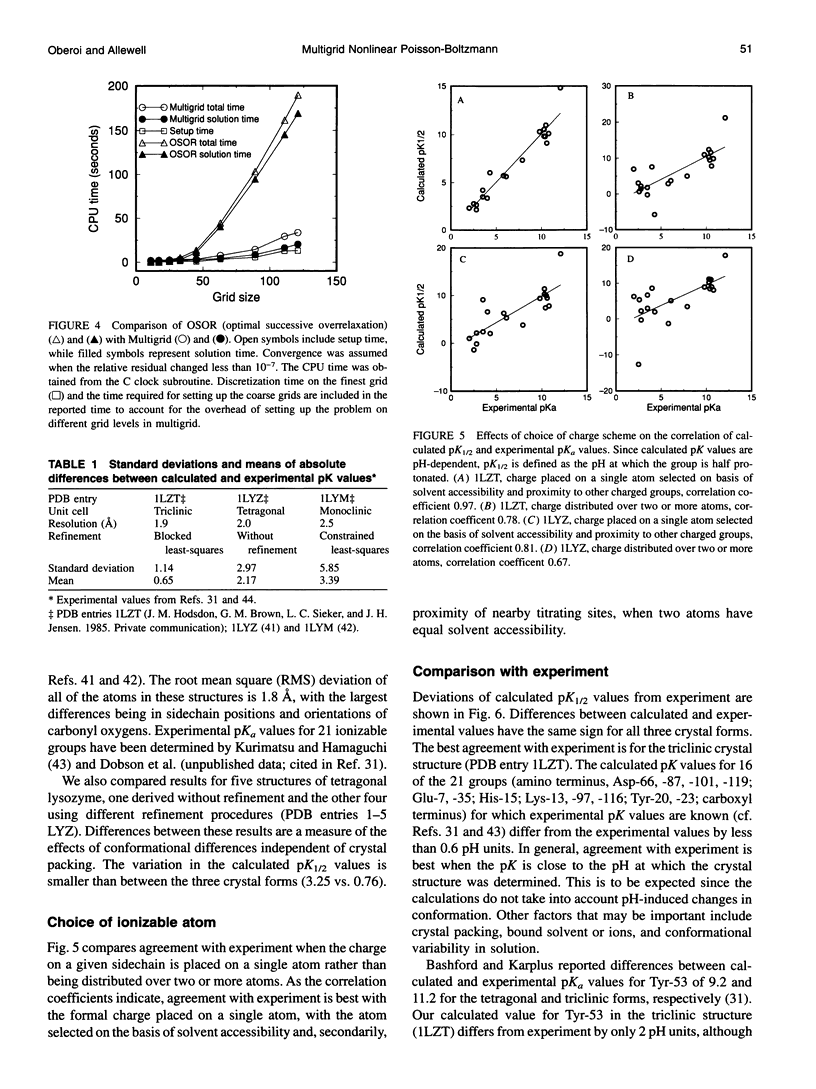

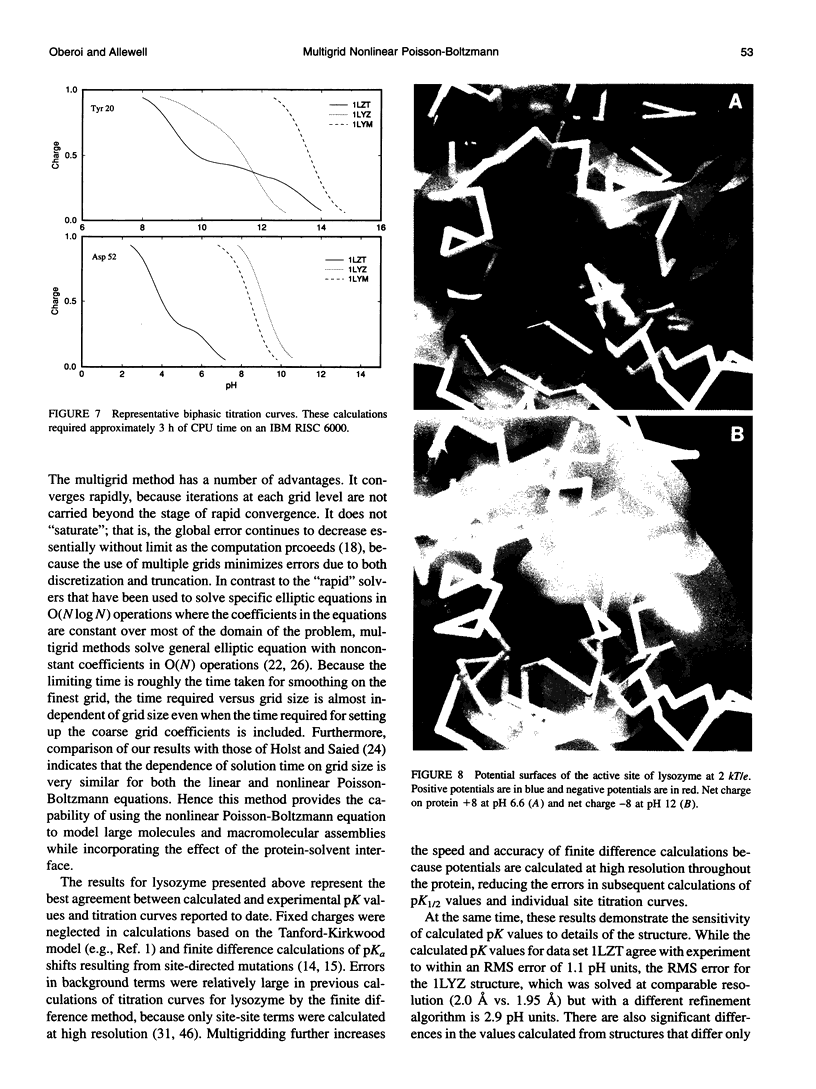


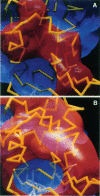
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allewell N. M., Oberoi H. Electrostatic effects in protein folding, stability, and function. Methods Enzymol. 1991;202:3–19. doi: 10.1016/0076-6879(91)02003-r. [DOI] [PubMed] [Google Scholar]
- Bashford D., Karplus M. pKa's of ionizable groups in proteins: atomic detail from a continuum electrostatic model. Biochemistry. 1990 Nov 6;29(44):10219–10225. doi: 10.1021/bi00496a010. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Beroza P., Fredkin D. R., Okamura M. Y., Feher G. Protonation of interacting residues in a protein by a Monte Carlo method: application to lysozyme and the photosynthetic reaction center of Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5804–5808. doi: 10.1073/pnas.88.13.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Diamond R. Real-space refinement of the structure of hen egg-white lysozyme. J Mol Biol. 1974 Jan 25;82(3):371–391. doi: 10.1016/0022-2836(74)90598-1. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. H. Calculation of electrostatic potentials in an enzyme active site. Nature. 1987 Nov 5;330(6143):84–86. doi: 10.1038/330084a0. [DOI] [PubMed] [Google Scholar]
- Gilson M. K., Honig B. H. Energetics of charge-charge interactions in proteins. Proteins. 1988;3(1):32–52. doi: 10.1002/prot.340030104. [DOI] [PubMed] [Google Scholar]
- Harvey S. C. Treatment of electrostatic effects in macromolecular modeling. Proteins. 1989;5(1):78–92. doi: 10.1002/prot.340050109. [DOI] [PubMed] [Google Scholar]
- Jayaram B., Sharp K. A., Honig B. The electrostatic potential of B-DNA. Biopolymers. 1989 May;28(5):975–993. doi: 10.1002/bip.360280506. [DOI] [PubMed] [Google Scholar]
- Klapper I., Hagstrom R., Fine R., Sharp K., Honig B. Focusing of electric fields in the active site of Cu-Zn superoxide dismutase: effects of ionic strength and amino-acid modification. Proteins. 1986 Sep;1(1):47–59. doi: 10.1002/prot.340010109. [DOI] [PubMed] [Google Scholar]
- Kuramitsu S., Hamaguchi K. Analysis of the acid-base titration curve of hen lysozyme. J Biochem. 1980 Apr;87(4):1215–1219. [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Matthew J. B., Gurd F. R. Calculation of electrostatic interactions in proteins. Methods Enzymol. 1986;130:413–436. doi: 10.1016/0076-6879(86)30019-3. [DOI] [PubMed] [Google Scholar]
- Matthew J. B., Gurd F. R., Garcia-Moreno B., Flanagan M. A., March K. L., Shire S. J. pH-dependent processes in proteins. CRC Crit Rev Biochem. 1985;18(2):91–197. doi: 10.3109/10409238509085133. [DOI] [PubMed] [Google Scholar]
- Northrup S. H., Wensel T. G., Meares C. F., Wendoloski J. J., Matthew J. B. Electrostatic field around cytochrome c: theory and energy transfer experiment. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9503–9507. doi: 10.1073/pnas.87.23.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C. H. Building models of globular protein molecules from their amino acid sequences. I. Theory. J Mol Biol. 1982 Feb 15;155(1):53–62. doi: 10.1016/0022-2836(82)90491-0. [DOI] [PubMed] [Google Scholar]
- Rogers N. K. The modelling of electrostatic interactions in the function of globular proteins. Prog Biophys Mol Biol. 1986;48(1):37–66. doi: 10.1016/0079-6107(86)90009-x. [DOI] [PubMed] [Google Scholar]
- Sharp K. A., Honig B. Electrostatic interactions in macromolecules: theory and applications. Annu Rev Biophys Biophys Chem. 1990;19:301–332. doi: 10.1146/annurev.bb.19.060190.001505. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Hayes F. R., Russell A. J., Thomas P. G., Fersht A. R. Prediction of electrostatic effects of engineering of protein charges. Nature. 1987 Nov 5;330(6143):86–88. doi: 10.1038/330086a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nakamura H., Wada A. Electrostatic forces in two lysozymes: calculations and measurements of histidine pKa values. Biopolymers. 1992 Aug;32(8):897–909. doi: 10.1002/bip.360320802. [DOI] [PubMed] [Google Scholar]
- Tanford C., Roxby R. Interpretation of protein titration curves. Application to lysozyme. Biochemistry. 1972 May 23;11(11):2192–2198. doi: 10.1021/bi00761a029. [DOI] [PubMed] [Google Scholar]
- Warshel A., Russell S. T. Calculations of electrostatic interactions in biological systems and in solutions. Q Rev Biophys. 1984 Aug;17(3):283–422. doi: 10.1017/s0033583500005333. [DOI] [PubMed] [Google Scholar]
- Warwicker J. Continuum dielectric modelling of the protein-solvent system, and calculation of the long-range electrostatic field of the enzyme phosphoglycerate mutase. J Theor Biol. 1986 Jul 21;121(2):199–210. doi: 10.1016/s0022-5193(86)80093-5. [DOI] [PubMed] [Google Scholar]
- Wendoloski J. J., Matthew J. B. Molecular dynamics effects on protein electrostatics. Proteins. 1989;5(4):313–321. doi: 10.1002/prot.340050407. [DOI] [PubMed] [Google Scholar]
- Yang A. S., Gunner M. R., Sampogna R., Sharp K., Honig B. On the calculation of pKas in proteins. Proteins. 1993 Mar;15(3):252–265. doi: 10.1002/prot.340150304. [DOI] [PubMed] [Google Scholar]