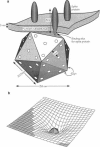
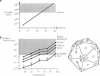
Abstract
How does a virus bud from the plasma membrane of its host? Here we investigate several possible rate-limiting processes, including thermal fluctuations of the plasma membrane, hydrodynamic interactions, and diffusion of the glycoprotein spikes. We find that for bending moduli greater than 3 x 10(-13) ergs, membrane thermal fluctuations are insufficient to wrap the viral capsid, and the mechanical force driving the budding process must arise from some other process. If budding is limited by the rate at which glycoprotein spikes can diffuse to the budding site, we compute that the budding time is 10-20 min, in accord with the experimentally determined upper limit of 20 min. In light of this, we suggest some alternative mechanisms for budding and provide a rationale for the observation that budding frequently occurs in regions of high membrane curvature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batenburg A. M., de Kruijff B. Modulation of membrane surface curvature by peptide-lipid interactions. Biosci Rep. 1988 Aug;8(4):299–307. doi: 10.1007/BF01115220. [DOI] [PubMed] [Google Scholar]
- Bo L., Waugh R. E. Determination of bilayer membrane bending stiffness by tether formation from giant, thin-walled vesicles. Biophys J. 1989 Mar;55(3):509–517. doi: 10.1016/S0006-3495(89)82844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989 Jul;56(1):151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Bostick D. A., Schram R., Waite M. R. The ratio of plasma membrane cholesterol to phospholipid and the inhibition of Sindbis virus maturation by low NaCl medium. J Gen Virol. 1985 May;66(Pt 5):1171–1177. doi: 10.1099/0022-1317-66-5-1171. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Warren G., Quinn P., Mathieu-Costello O., Hoppeler H. Density of newly synthesized plasma membrane proteins in intracellular membranes. I. Stereological studies. J Cell Biol. 1984 Jun;98(6):2133–2141. doi: 10.1083/jcb.98.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D., Hochmuth R. M. A sensitive measure of surface stress in the resting neutrophil. Biophys J. 1992 Jun;61(6):1664–1670. doi: 10.1016/S0006-3495(92)81970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster G. F., Cheng L. Y., Moore H. P., Perelson A. S. Vesicle formation in the Golgi apparatus. J Theor Biol. 1989 Dec 19;141(4):463–504. doi: 10.1016/s0022-5193(89)80231-0. [DOI] [PubMed] [Google Scholar]
- Petersen N. O., Johnson D. C., Schlesinger M. J. Scanning fluorescence correlation spectroscopy. II. Application to virus glycoprotein aggregation. Biophys J. 1986 Apr;49(4):817–820. doi: 10.1016/S0006-3495(86)83710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Kinetics of incorporation of structural proteins into Sindbis virions. J Virol. 1969 Apr;3(4):369–375. doi: 10.1128/jvi.3.4.369-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980 Sep;50(1):1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- Vaux D. J., Helenius A., Mellman I. Spike--nucleocapsid interaction in Semliki Forest virus reconstructed using network antibodies. Nature. 1988 Nov 3;336(6194):36–42. doi: 10.1038/336036a0. [DOI] [PubMed] [Google Scholar]
- Yoneda M. The compression method for determining the surface force. Methods Cell Biol. 1986;27:421–434. doi: 10.1016/s0091-679x(08)60362-3. [DOI] [PubMed] [Google Scholar]