Abstract
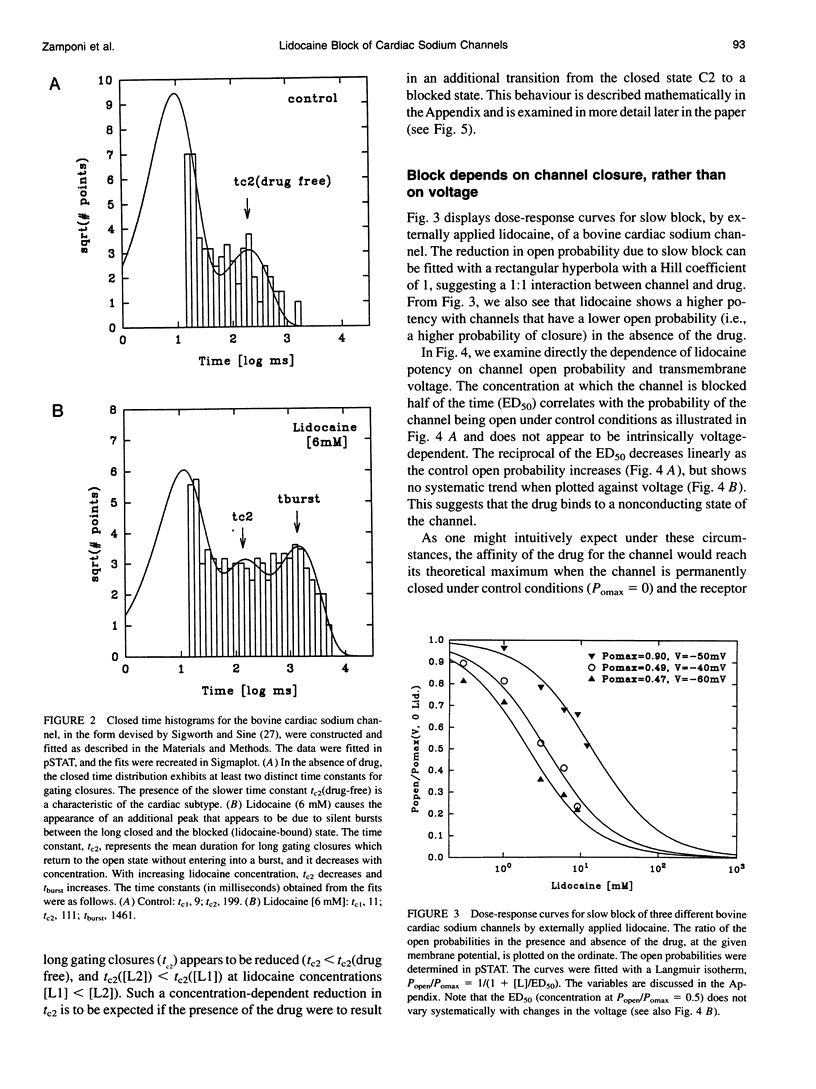
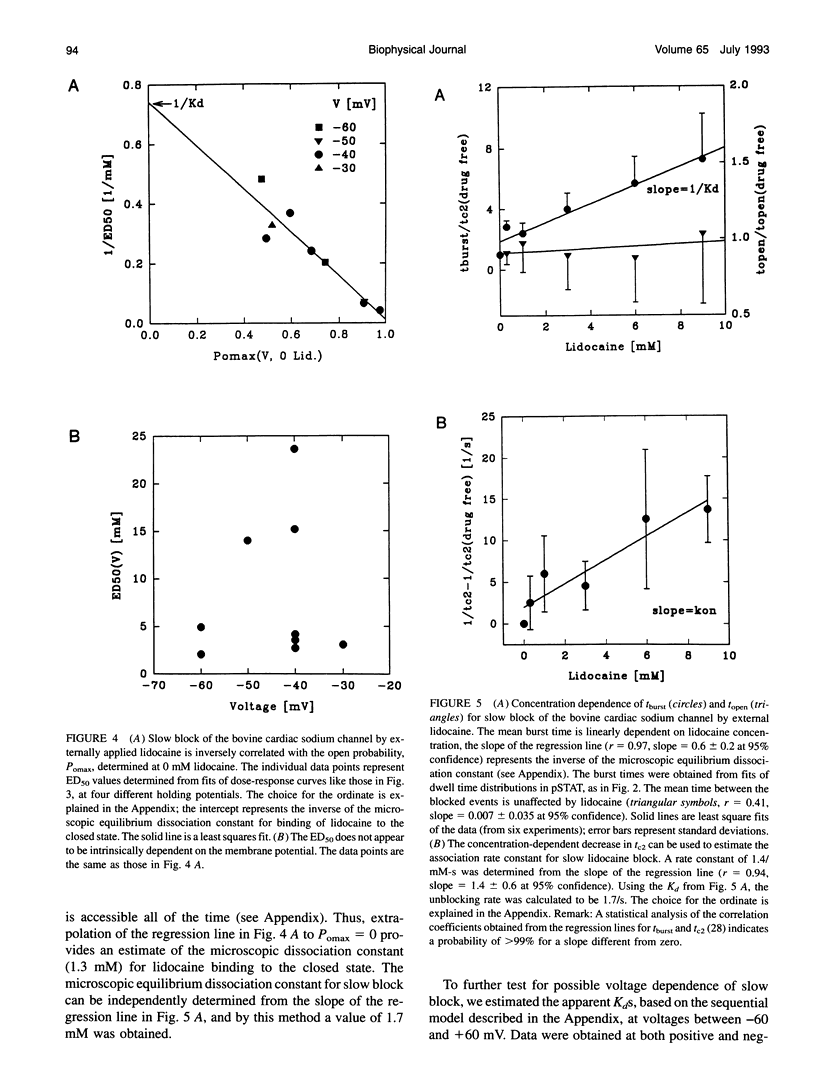
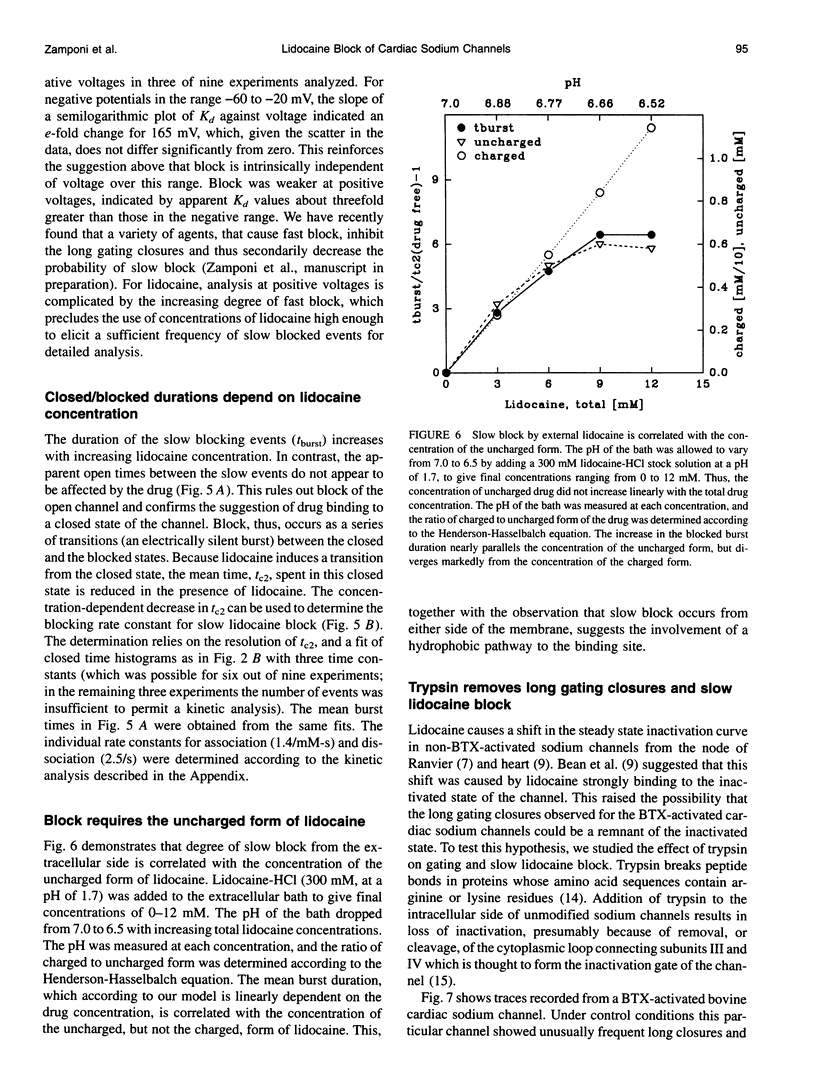
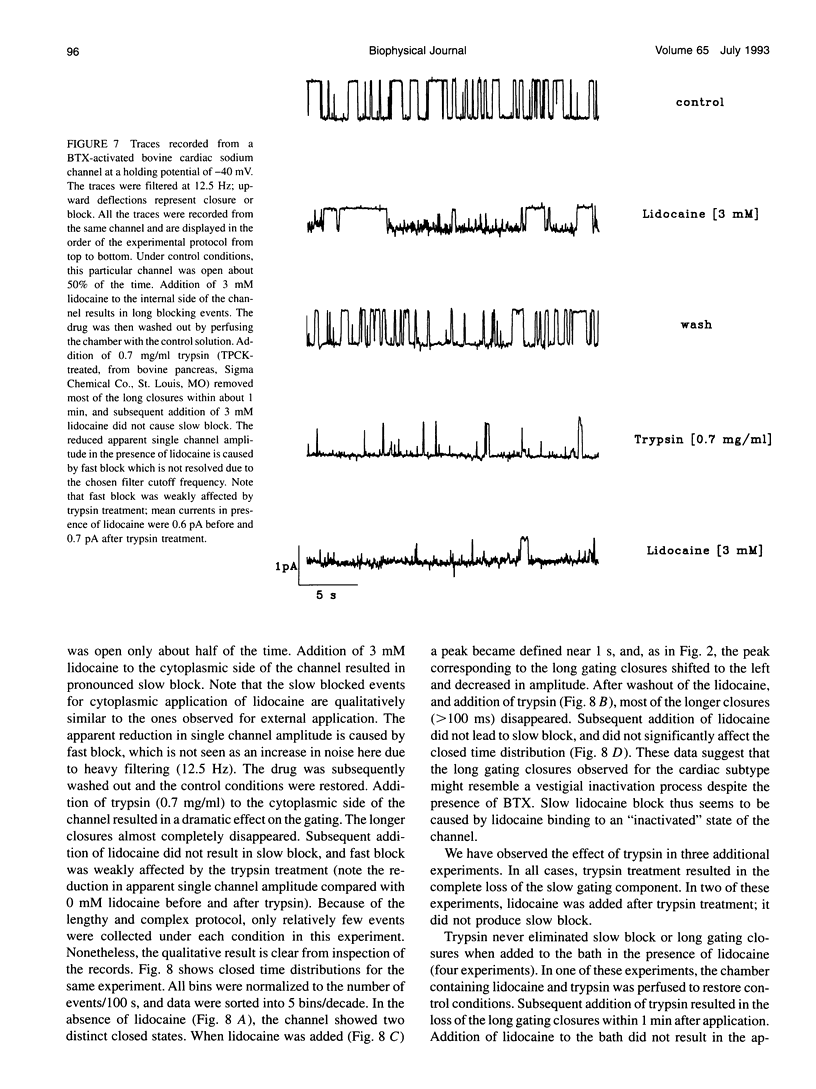
We have identified two kinetically distinct modes of block, by lidocaine, of cardiac sodium channels, activated by batrachotoxin and incorporated into planar lipid bilayers. Here, we analyze the slow blocking mode which appears as a series of nonconducting events that increase in frequency and duration with increasing lidocaine concentrations. This type of block occurred rarely, if at all, for the skeletal muscle sodium channel subtype. Kinetic analysis showed that a linear open-closed-blocked model is sufficient to account for the major features of our data. Slow block occurs from a long closed state that is a distinguishing characteristic of cardiac channels under these conditions. Slow block showed no significant voltage dependence in the range of -60 to -20 mV for which the detailed kinetic analysis was performed, and was not elicited by application of the permanently charged lidocaine derivative QX-314. By contrast, the fast block, described in the companion paper, results from drug binding to the open state, and is similar for cardiac and skeletal muscle sodium channels. Application of trypsin to the cytoplasmic end of the channel eliminates both the spontaneous, long, gating closures and slow block. Thus, the lidocaine-sensitive closed state of batrachotoxin-activated cardiac sodium channels exhibits a protease susceptibility resembling that of the inactivated state of unmodified sodium channels. It is the slow block caused by lidocaine binding to this closed state that underlies the channel-subtype specificity of lidocaine action in our experiments.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert L. A., Fozzard H. A., Hanck D. A., Makielski J. C. Is there a second external lidocaine binding site on mammalian cardiac cells? Am J Physiol. 1989 Jul;257(1 Pt 2):H79–H84. doi: 10.1152/ajpheart.1989.257.1.H79. [DOI] [PubMed] [Google Scholar]
- Baumgarten C. M., Makielski J. C., Fozzard H. A. External site for local anesthetic block of cardiac Na+ channels. J Mol Cell Cardiol. 1991 Feb;23 (Suppl 1):85–93. doi: 10.1016/0022-2828(91)90027-j. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W., Follmer C. H., Ten Eick R. E., Hondeghem L. M., Yeh J. Z. Evidence for two components of sodium channel block by lidocaine in isolated cardiac myocytes. Circ Res. 1988 Nov;63(5):869–878. doi: 10.1161/01.res.63.5.869. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W. Modification of Na channel inactivation by alpha-chymotrypsin in single cardiac myocytes. Pflugers Arch. 1990 Sep;417(1):48–57. doi: 10.1007/BF00370768. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S. Inactivation modifiers of Na+ currents and the gating of rat brain Na+ channels in planar lipid membranes. Pflugers Arch. 1991 Nov;419(5):514–521. doi: 10.1007/BF00370798. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Krafte D. S., Volberg W. A., Dillon K., Ezrin A. M. Expression of cardiac Na channels with appropriate physiological and pharmacological properties in Xenopus oocytes. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4071–4074. doi: 10.1073/pnas.88.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makielski J. C., Alpert L. A., Hanck D. A. Two components of use-dependent block of sodium current by lidocaine in voltage clamped cardiac Purkinje cells. J Mol Cell Cardiol. 1991 Feb;23 (Suppl 1):95–102. doi: 10.1016/0022-2828(91)90028-k. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Clarkson C., Hondeghem L. Lidocaine blocks open and inactivated cardiac sodium channels. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):224–231. doi: 10.1007/BF00165809. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Uehara A., Guo X., Heiny J. Isochannels and blocking modes of voltage-dependent sodium channels. Ann N Y Acad Sci. 1986;479:269–292. doi: 10.1111/j.1749-6632.1986.tb15575.x. [DOI] [PubMed] [Google Scholar]
- Rogart R. B., Cribbs L. L., Muglia L. K., Kephart D. D., Kaiser M. W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin J., Kyle J. W., Chen M., Bell P., Cribbs L. L., Fozzard H. A., Rogart R. B. A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science. 1992 May 22;256(5060):1202–1205. doi: 10.1126/science.256.5060.1202. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer C. F., Nesterenko V. V., Undrovinas A. I., Grant A. O., Rosenshtraukh L. V. Lidocaine blockade of continuously and transiently accessible sites in cardiac sodium channels. J Mol Cell Cardiol. 1991 Feb;23 (Suppl 1):73–83. doi: 10.1016/0022-2828(91)90026-i. [DOI] [PubMed] [Google Scholar]
- Strichartz G. Molecular mechanisms of nerve block by local anesthetics. Anesthesiology. 1976 Oct;45(4):421–441. doi: 10.1097/00000542-197610000-00012. [DOI] [PubMed] [Google Scholar]
- Wang G. K., Wang S. Y. Inactivation of batrachotoxin-modified Na+ channels in GH3 cells. Characterization and pharmacological modification. J Gen Physiol. 1992 Jan;99(1):1–20. doi: 10.1085/jgp.99.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z. Sodium inactivation mechanism modulates QX-314 block of sodium channels in squid axons. Biophys J. 1978 Nov;24(2):569–574. doi: 10.1016/S0006-3495(78)85403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., Doyle D. D., French R. J. Fast lidocaine block of cardiac and skeletal muscle sodium channels: one site with two routes of access. Biophys J. 1993 Jul;65(1):80–90. doi: 10.1016/S0006-3495(93)81042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


