Abstract
Elevated hyaluronan biosynthesis and matrix deposition correlates with cell proliferation and migration. We ectopically expressed three isoforms of hyaluronan synthase (HAS1, HAS2, or HAS3) in nontransformed rat 3Y1 cells and observed a de novo, massive formation of a hyaluronan matrix that resulted in a partial loss of contact-mediated inhibition of cell growth and migration. All three HAS transfectants showed an enhanced motility in scratch wound assays, and a significant increase in their confluent cell densities. In high-density cultures, the HAS transfectants had a fibroblastic cell shape and markedly formed overlapping cell layers. This phenotype was more pronounced in the HAS2 transfectants than HAS1 or HAS3 transfectants, and occurred with significant alterations in the microfilament organization and N-cadherin distribution at the cell–cell border. Inhibition of a phosphatidylinositol 3-kinase (PI3-kinase) pathway resulted in reacquisition of the normal phenotype of HAS2 transfectants, suggesting that the intracellular PI3-kinase signaling regulates diminution of contact inhibition induced by formation of the massive hyaluronan matrix. Our observations suggest that hyaluronan and its matrix can modulate contact inhibition of cell growth and migration, and provide evidence for functional differences between hyaluronan synthesized by the different HAS proteins.
Hyaluronan (HA) is a linear polysaccharide composed of a repeating disaccharide: N-acetyl-d-glucosamine-β(1→4)-d-glucuronic acid-β(1→3), and is widely present as a major component of extracellular matrix in most vertebrate tissues (1, 2). HA is synthesized by three HA synthase isoforms: HAS1, HAS2, and HAS3 (3, 4). The three HAS genes have distinct expression patterns during mouse development (5), and their products have significantly different enzymatic properties and roles in the formation of the HA matrix (6).
HA synthesis and matrix formation may have a role in the development, progression and pathogenesis of cancer. For example, the rate of HA synthesis is enormously increased when oncogenic viruses transform fibroblasts (7, 8), and elevated levels of HA are associated with the hyperproliferative and malignant phenotypes in melanomas and various carcinomas (9–11).
Studies done with cultured cancer cells showed that overproduction of HA enhances their anchorage-independent growth, tumorigenicity, and metastatic potential (12, 13), suggesting an important role for HA in tumor growth and malignant progression. However, it was unclear whether the overproduction could induce a malignant transformation of nontransformed cells.
We overexpressed the three HAS isoforms to test the ability of HA to transform nontransformed cells. We also investigated whether the three HAS isoforms are functionally distinct in nontransformed cells.
Materials and Methods
Cell Culture and Transfection.
3Y1 1-B6 cells were obtained from Riken Cell Bank (Tsukuba, Japan). The 3Y1 cells were cultured in DMEM containing 10% FCS and 2 mM l-glutamine (growth medium). Stable transfectants were established as previously described (6) and were routinely cultured in growth medium containing 0.5 mg/ml of G418.
Particle Exclusion Assays.
Fixed sheep erythrocytes (Inter-Cell Technologies, Hopewell, NJ) were reconstituted in PBS to a density of 5 × 108 cells/ml and used for the particle exclusion assay as described previously (6). HA matrices were visualized by adding 1 × 107 erythrocytes to the growth medium and viewing under an Olympus (New Hyde Park, NY) IMT-2 inverted phase-contrast microscope. The HA pericellular coat to cell area ratios were determined by tracing the digitized image by using NIH image (ver. 1.57) software on a computer. All measurements were described in a previous paper (6).
Detection of the Expression of FLAG-Tagged Recombinant HAS Proteins.
Western blotting was performed according to the manufacturer's instructions by using anti-FLAG peptide antibody M5 (Eastman Kodak) followed by anti-mouse IgG antibody conjugated with peroxidase. Immune complexes were detected by exposure for 10 s by using the ECL detection system (Amersham Pharmacia Biotech).
Determination of HA Concentrations by Competitive ELISA-Like Assay.
Exponentially growing and confluent cultures were cultured in fresh culture medium for 24 h, and the conditioned medium was recovered. The HA content of the conditioned medium was measured by a competitive ELISA-like assay as described previously (6). Briefly, the conditioned medium was mixed with biotinylated HA binding protein (b-HABP) and incubated at 4°C for 20 h. The mixture was added to the HA-conjugated 96-wells and then incubated for 6 h at room temperature. Alkaline phosphatase-conjugated streptavidin was used as secondary probe, and the enzymatic activity was measured by using p-nitrophenyl phosphate as the substrate. HA contents were calculated by using a standard curve.
HA Synthase Assays.
HA synthase activity was monitored by using UDP-[14C]GlcA [281 mCi (1 Ci = 37 GBq)/mmol; NEN; GlcA = glucuronic acid residue] as described previously (6). Briefly, the crude membrane fractions of HAS transfectants were resuspended and incubated at 37°C for 1 h in 0.2 ml of 25 mM Hepes-NaOH, pH 7.1/5 mM DTT/15 mM MgCl2/0.1 mM UDP-GlcNAc (Sigma)/2 μM UDP-GlcA (Nacalai Tesque, Kyoto)/2 μCi of UDP-[14C]GlcA. Reactions were terminated by adding of SDS to 2% (wt/vol). The incorporation of radioactivity into high molecular mass HA was measured by descending paper chromatography by using Whatman no. 3MM paper developed in 1 M ammonium acetate (pH 5.5) and ethanol (65:35). The amounts of radioactivity in the origins were measured by liquid scintillation counting. The synthase activity was determined by calculating the amounts of GlcA incorporated by using known specific radioactivities.
Cell Proliferation Assay.
Cell proliferation assays were performed by using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). Cells were plated in 96-well plates at 1 × 104 cells per well and cultured in the growth medium. At the indicated time points, the cell numbers in triplicate wells were measured as the absorbance (450 nm) of reduced WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt).
Repair of Wounded Cell Monolayers.
Confluent monolayers of cells were maintained in serum-containing growth medium for at least 6 days, and then in serum-free medium for 24 h. A 200-μl plastic pipette tip was used to scratch the monolayers. The wounded cells were then cultured in serum-free medium for an additional 6 h, and photographed under an inverted phase contrast microscope. Three different points were marked on the plate, and the lengths between the scratch wound's edge were measured before and after cell migration at these points. The mean migration distance (μm) was calculated by subtracting the length after 6 h from that at 0 h.
Microfilament Staining and Immunostaining.
Cells were cultured in Lab-Tek chamber slides (Nalge Nunc) until confluent. The confluent cells were fixed with 4% paraformaldehyde in PBS for 15 min and then permeabilized for 10 min with 0.5% Triton X-100 in PBS. Fixed and permeabilized cells were incubated with rhodamine-phalloidin (Sigma) in a moist chamber for 20 min, and the chamber slides were mounted with a SlowFade Light Antifade Kit (Molecular Probes). The fixed and permeabilized cells were incubated with pan anti-cadherin antibody (Sigma), anti-N-cadherin antibody or β-catenin antibody (Transduction Laboratories, Lexington, KY) in a moist chamber for 1 h, and then incubated with FITC-conjugated anti-rabbit antibody or anti-mouse antibody (Amersham Pharmacia Biotech) for 1 h. The fixed cells were incubated with biotinylated HA binding protein in a moist chamber for 1 h, and then incubated with FITC-conjugated streptavidin (Amersham Pharmacia Biotech) for 1 h. The fluorescent images were photographed under a fluorescent microscope.
Scanning Electron Microscopy.
Cell layers were grown on coverslips, washed with PBS, and fixed with 2.5% (wt/vol) paraformaldehyde, 2% (wt/vol) glutaraldehyde in 50 mM phosphate buffer for 1 h on ice. Cell layers were postfixed with 1% osmium tetroxide for 2 h. After dehydration in a graded series of ethanol followed by isoamyl acetate, the specimens were lyophilized, coated with gold, and examined under a Hitachi scanning electron microscope model S-4700.
BrdUrd Incorporation Assay.
The cells were labeled by incubation for 24 h in 10 μM BrdUrd in fresh medium, fixed in acid-ethanol (5% acetic acid and 5% distilled water in ethanol) for 30 min at room temperature, and incubated with mouse anti-BrdUrd monoclonal antibody (BD PharMingen) for 1 h at room temperature. After incubation with horseradish peroxidase-labeled anti-mouse IgG (Amersham Pharmacia Biotech) at room temperature for 30 min, the incorporation of BrdUrd into nuclei was visualized by staining with 3,3′-diaminobenzidine tetrahydrochloride. The percentage of cells incorporating BrdUrd was calculated from two independent experiments that measured the mean of BrdUrd-positive cells in three different microscopic fields. Arrested cells were challenged with 1 mg/ml of human umbilical vein HA with a molecular mass of more than 106 Da (endotoxin content, <0.001 endotoxin units/mg) and the HA fragments that have molecular masses of 3,800 and 5,500 Da (endotoxin contents, 0.103 endotoxin units/mg and 0.001 endotoxin units/mg, respectively); then the percentage of cells incorporating BrdUrd was calculated as above.
Cell cycle profiles were analyzed by flow cytometry as described previously (14). Briefly, growing and confluent cells were pulsed with 10 μM BrdUrd for a further 24 h. Cells were fixed with 70% ethanol, rinsed with PBS, and resuspended in 1 ml of cold 0.1 N HCl/0.7% Triton X-100 on ice. After being rinsed once with PBS, they were heated for 8 min at 95°C in 0.5 ml of 3.2 mM HCl and placed immediately on ice. The cells were rinsed twice with HNFN buffer (10 mM Hepes/150 mM NaCl/4% FCS/0.1% NaN3) containing 0.5% Tween 20, and once with HNFN alone. The cells were labeled with anti-BrdUrd monoclonal antibody and then with FITC-conjugated anti-mouse IgG antibody. The stained cells were resuspended in 1 ml of PI solution (50 μg/ml propidium iodide/10 μg/ml ribonuclease A in PBS), and left for 1 h (4°C) in the dark before analysis on an Epics Elite V flow cytometer (Coulter).
Soft Agar Growth Assay and Tumor Formation.
Six-well plates were coated with 1 ml of 0.6% agarose in growth medium. Then, 2 × 105, 2 × 104, and 2 × 103 cells from each cell pool were suspended in 0.2 ml of growth medium. Each cell suspension was mixed with 0.3% agarose growth medium (52°C) and added at 2 ml in duplicate to 0.6% agarose-coated wells. The agarose solidified at room temperature within 20 min, and 3 ml of growth medium was added to each well. The medium was changed every 3 days for 1 month, and the colonies were counted every week. Six-week-old female BALB/c nu/nu mice were injected s.c. in the left flank with 0.1 ml of a suspension of 2 × 106 cells. The appearance of visible tumors was monitored for 6 months.
Results
Ectopic Expression of HAS Proteins Induces Overproduction of HA and Abnormal Accumulation of HA Matrix.
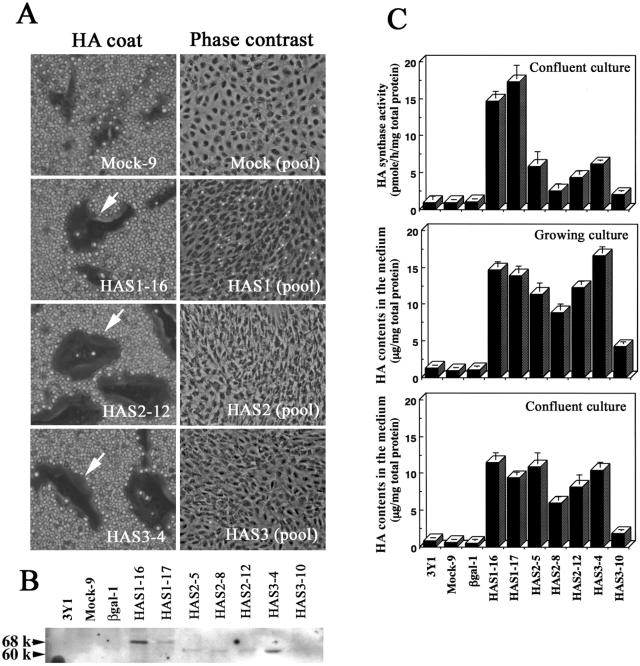
We studied the effects of HA on cell growth and transformation by transfecting and expressing different HA synthases in the nontransformed, immortal rat 3Y1 cell line (15). We observed that overexpression of HAS1, HAS2, or HAS3 in 3Y1 cells caused an overproduction of HA and de novo formation of an HA pericellular coat (Fig. 1). Previous and the present experiments showed that the HA pericellular coat of the HAS2 transfectants was obviously larger than that of the HAS1 or HAS3 transfectants, even though the HAS1 transfectant produced an equal or greater amount of HA into the conditioned medium (ref. 6, Fig. 1 A and C). We detected only FLAG-tagged recombinant protein in the HAS transfectants, which means that the overproduction of HA in the transfectants is attributable to the ectopic expression of HAS proteins (Fig. 1B). HA synthesis is known to be down-regulated in growth-arrested cells, but in our transfectants the synthesis and HA accumulation in the medium were maintained at levels severalfold higher than in control transfectants, even after the cells reached confluence (Fig. 1C). Such significant differences in the formation of the HA matrix prompted us to investigate the biological meaning of the different HA synthesized by their respective HAS.
Figure 1.
Effects of HAS overexpression on cell density and morphology of confluent rat 3Y1 cells. (A) Rat 3Y1 fibroblasts were transfected with control vector or with HAS expression vectors, and clonal transfectants were established (6). HA coat formations (arrows) were detected by the particle exclusion assay. The parental cells were transfected with each HAS cDNA, and the G418-resistant transfectants were pooled. The transfectants were plated in 10-cm tissue culture dishes at 5 × 105 cells/dish and cultured for 14 days. The pooled HAS transfectants showed a spindle-like morphology and overlapping cell layers. (B) Western blotting analyses of membrane fractions isolated from the transfectants expressing FLAG-tagged HAS protein. Arrowheads indicate protein bands of the FLAG-tagged HAS1 (68 kDa), HAS2 (60 kDa), and HAS3 proteins (60 kDa). (C) HA synthase activities of confluent cultures were determined as described in Materials and Methods. The HA contents of the conditioned medium of exponentially growing and confluent cultures were measured by a competitive ELISA-like assay as described in Materials and Methods. Column, mean values of three determinations; bars, SD.
Overproduction of HA and Abnormal Accumulation of HA Matrix Promotes Proliferation and Migration of Confluent Cells.
We examined the possibility that endogenously overproduced HA acts as a modulator of cell growth. A growth curve at low cell density showed no obvious growth stimulation or suppression in 3Y1 cells overexpressing HAS (data not shown). After reaching confluence, the parental and control cells became quiescent by contact inhibition, but all HAS transfectants gradually increased their saturation density (Table 1). The confluent cultures were then scratch wounded and examined for the rate of wound repair. The HAS transfectants migrated into a scratch wound more rapidly than the parental 3Y1 cells and control transfectants (Table 1). These phenotypes were intermediate for HAS1 and HAS3 cells, and most pronounced in the HAS2 transfectants, suggesting a close relationship between the levels of HA matrix formation and contact inhibition of cell motility.
Table 1.
Saturation cell density and cell motility of HAS transfectants
| Clones | Saturation cell density* | Cell motility,† μm ± SD | Cell overlap |
|---|---|---|---|
| 3Y1 | 1.00 | 35.0 ± 11.0 | − |
| Mock-9 | 1.04 ± 0.04 | 32.2 ± 10.5 | − |
| βgal-1 | 1.03 ± 0.08 | 32.3 ± 7.7 | − |
| HAS1–16 | 1.16 ± 0.16‡ | 44.5 ± 7.8§ | + |
| HAS1–17 | 1.13 ± 0.2§ | 45.3 ± 4.3§ | + |
| HAS2–5 | 1.23 ± 0.03‡ | 56.3 ± 7.3§ | + |
| HAS2–8 | 1.19 ± 0.06§ | 52.3 ± 5.3§ | + |
| HAS2–12 | 1.25 ± 0.13‡ | 58.0 ± 11.8‡ | + |
| HAS3–4 | 1.18 ± 0.09‡ | 52.3 ± 9.2‡ | + |
| HAS3–10 | 1.16 ± 0.06§ | 47.7 ± 6.8§ | + |
For cell proliferation assay, cells were plated in 96-well plates at 1 × 104 cells per well and cultured for 14 days. Cell overlap was observed under the inverted phase contrast microscope.
Values shown are relative saturation density compared with 3Y1 (n = 3).
Values were expressed as mean ± SD of six experiments.
P < 0.01 vs. Mock-9.
P < 0.05 vs. Mock-9.
Formation of a Massive HA Matrix Induces Stratified Cell Layers and Alters Cytoskeletal Organization.
We generated stable transfectant pools to avoid any phenotypic artifacts that can result from the selection and propagation of individual clones derived from single transfected cells. After each transfection, we combined the G418-resistant colonies. The HA pericellular coat was clearly present on more than 80% of the HAS transfectants, whereas coat formation was not evident in the control cells (data not shown). The parental and control cells were monolayers of nonoverlapping cells in confluent cultures, whereas the pooled HAS transfectants showed a spindle-like morphology and an overlapping cell layer, characteristic of cellular transformation (Fig. 1A). Under the phase-contrast microscope, these features of morphological transformation were more pronounced in the cells expressing HAS2 than either the HAS1 or the HAS3 transfectants (Fig. 1A). The HAS1 and HAS3 transfectants form less HA matrix, and we observed a number of floating cells in their cultures, suggesting that the massive HA matrix serves as a scaffold during formation of a stratified cell layer.
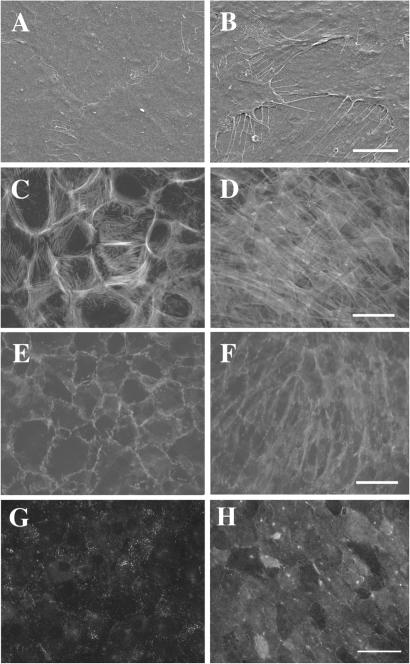
Scanning electron microscopy revealed a number of filopodial protrusions at the cell edges of the HAS2 transfectants (Fig. 2B). In contrast, the quiescent control transfectants formed tight junctions at their cell borders (Fig. 2A). Rhodamine-phalloidin staining showed a significant alteration of the actin filament organization in HAS2 transfectants as compared with control cells (Fig. 2 C and D). The confluent HAS2 transfectants changed from having mainly cortical actin filaments to predominantly actin stress fibers. On the other hand, HAS1 and HAS3 transfectants showed a moderate alteration in their actin organization (data not shown). Immunofluorescence experiments revealed that the distribution of cadherin at the intercellular boundaries was significantly altered in the HAS2 transfectants (Fig. 2F). In contrast, cadherin mainly accumulated at the intercellular boundaries of control transfectants and the distribution was indistinguishable from that of the parental 3Y1 cells (Fig. 2E).
Figure 2.
Morphological and cytoskeletal alterations of confluent HAS2 transfectants. Confluent cultures of control transfectants (A, C, E, and G) and HAS2 transfectants (B, D, F, and H) were analyzed by scanning electron microscope (A and B) and by staining with rhodamine-phalloidin (C and D), pan anti-cadherin antibody (E and F) or biotinylated HA binding protein (HABP; G and H). Scanning electron micrographs revealed a number of filopodial protrusions at the cell edges of the HAS2 transfectants (B). The confluent HAS2 transfectants changed their actin filament organization from cortical actin filaments to actin stress fibers (D). The distribution of cadherin at the intercellular boundaries was significantly altered in the HAS2 transfectants (F). Bars = 5 μm (A and B) and 20 μm (C–H).
Overproduction of HA in Nontransformed Cells Does Not Enhance Their Anchorage-Independent Growth or Ability to Form Subcutaneous Tumors.
To assess the oncogenic capacity of HAS in vitro, cells were suspended in 0.3% agar and allowed to grow for 1 month. This soft agar assay showed no obvious colony formation in either of the HAS transfectants and control cells. We s.c. injected these HAS transfectants into nude mice, and did not observe tumor formation at the site of injection within 6 months.
Cell Cycle Progression of Confluent Cells Is Associated with Overproduction of HA.
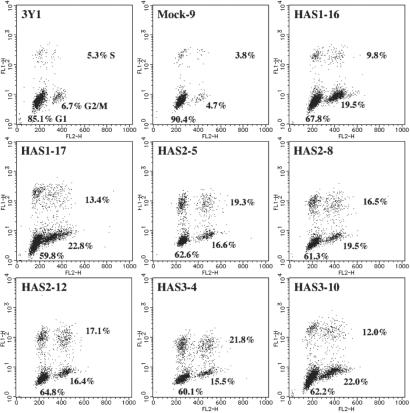
We used the BrdUrd incorporation assay to monitor the growth patterns of HAS transfectants. When the exponentially growing cells were stained immunocytochemically, the percentage of BrdUrd-incorporating cells was found to be more than 15% for all of the HAS transfectants and control cells. After confluence, less than 3% of the control 3Y1 cells were found to be BrdUrd-incorporating cells. In contrast, we observed a significant increase in the percentage of BrdUrd-incorporating cells after the HAS2 transfectants reached confluence. To characterize the distribution of these cells in the cell cycle, the incorporation of BrdUrd into the confluent cells was analyzed by flow cytometry. Cell cycle profiles of the parental and control cells revealed a decrease in the proportion of S and G2/M cells and an increase in the proportion of G0/G1 cells (Fig. 3). In contrast, all HAS transfectant clones had a higher proportion of S phase and G2/M phase, and a lower percentage of G0/G1 phase, as compared with the control cells (Fig. 3).
Figure 3.
Cell cycle progression of contact-inhibited 3Y1 cells by HA overproduction. Cell cycle profiles were monitored by flow cytometry at 24 h after the pulsing of confluent cell cultures with BrdUrd. Cells labeled with BrdUrd were prepared as described in the Materials and Methods. All HAS transfectants had a high proportion of cells in S phase and G2/M phase, and a low percentage in G0/G1 phase, as compared with the control cells.
Exogenous Addition of HA Induces DNA Synthesis but Not Stratification in Confluent Cells.
To further confirm whether the cell cycle progression at high cell density was due to overproduction of HA, the 3Y1 cells were exposed to exogenous soluble HA over a certain period and then monitored for the incorporation of BrdUrd. When these arrested cells were challenged with 1 mg/ml of high molecular weight HA (molecular mass of more than 106 Da), the parental cells were partly released from their contact inhibition of growth and resumed their cell cycle progression. Less than 3% of the cells initiated DNA synthesis in the control culture without HA, whereas 10.8 ± 2.8% of cells had incorporated BrdUrd after HA stimulation. Because the physiological roles of HA vary depending on chain length, we also examined the effects of short HA fragments on the cell cycle progression. When these arrested cells were challenged with the HA fragments that have molecular masses of 3,800 and 5,500 Da, a weak but significant growth promotion was observed (7.3 ± 0.9% and 8.2 ± 2.1% BrdUrd incorporations, respectively). Interestingly, neither high nor low molecular weight HA had an effect on the formation of HA matrix, and neither induced a stratified cell layer.
To elucidate the effects of HA secreted in conditioned medium on cell proliferation, we exposed 3Y1 cells to the conditioned media of the HAS transfectants. When the parental cells were challenged with the conditioned media from 3Y1-mock-9 and β-galactosidase (βgal)-1 cells, the percentages of BrdUrd incorporation were 0.4 ± 0.1% and 1.3 ± 0.6%, respectively. In contrast, the incorporation of BrdUrd into parental cells was slightly but significantly increased after treatments with the conditioned media from HAS2–5, HAS2–8 and HAS2–12 cells (5.6 ± 1.9%, 3.7 ± 1.1%, and 5.8 ± 0.9%, respectively). Under the same experimental conditions, however, stratification of the cell layer at high cell density was not observed even after 2 weeks (data not shown). These results suggested that overproduction of HA plays roles in cell cycle regulation at high cell density and make it clear that, when the HA matrix accumulates abnormally, it significantly aids in the formation of a stratified cell layer.
Inhibition of the PI3-Kinase Pathway Resulted in Reacquisition of Normal Phenotype of the HAS2 Transfectants.
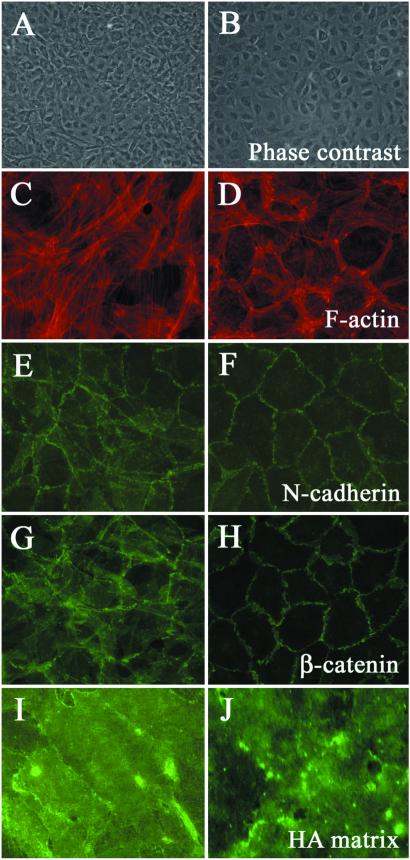
Intracellular signals regulate a variety of cellular processes, including proliferation, cell–cell communication, and transformation. Our recent study on the actions of the PI3-kinase inhibitor on HA-dependent cell locomotion has suggested that the activation of the PI3-kinase by the HA-CD44 interaction is required for the enhancement of HA-dependent cell locomotion (16). To test the effects of PI3-kinase inhibitors on the cellular transformation, HAS2 stable transfectants were treated with either LY294002 or wortmannin, potent inhibitors of PI3-kinase. Inhibition of the PI3-kinase resulted in a reacquisition of the normal phenotype of the HAS2 transfectants as demonstrated by the cell shape, actin cytoskeletal organization, and distributions of cell–cell machinery (Fig. 4). However, HA matrix was maintained on the HAS2 transfectants even after treatment with PI3-kinase inhibitors, suggesting that the HA-mediated activation of PI3-kinase has roles in regulating the stabilization of cell–cell adhesions and the contact inhibition of cell growth.
Figure 4.
Inhibition of the PI3-kinase pathway resulted in reacquisition of normal phenotype of the HAS2 transfectants. The HAS2 stable transfectants were cultured with (B, D, F, H, and J) or without (A, C, E, G, and I) 10 μM of LY294002, a PI3-kinase inhibitor, just after the cells reached confluence, and cultured for additional 5 days in the presence of the inhibitor. The HAS2 transfectants treated with the inhibitor showed normal morphology (B). Rhodamine-phalloidin staining of actin showed reappearance of pericellular actin rings in the HAS2 transfectants treated with the inhibitor (D). Immunofluorescent staining of N-cadherin and β-catenin in HAS2 transfectants demonstrated that PI3-kinase inhibition resulted in the accumulations of N-cadherin and β-catenin at the cell borders (F and H) whereas HA matrix was maintained on the HAS2 transfectants even after the treatment with LY294002 (J). Wortmannin (50 μM), a potent PI3-kinase inhibitor, also gave similar effects on the phenotypic changes of the HAS2 transfectants (data not shown).
Discussion
We established stable HAS transfectants in rat 3Y1 cells to examine the possible roles and modes of action of HA in cellular transformation. Here, we showed that the overexpression of HAS induces an abnormal accumulation of HA matrix that accounts for a partial loss of contact inhibition. Furthermore, we attempted to elucidate the functional differences between the three HAS isoforms and found that the phenotypes were most pronounced in the HAS2 transfectant while intermediate in nature in the HAS1 and HAS3 transfectants. As expected in our previous paper (6), these phenotypic differences may be explained by the differences in the enzymatic properties of the respective HAS. The current findings obtained by gene manipulations provide evidence for functional differences in the HA molecules synthesized by different HAS proteins.
Previous studies suggested possible roles for HA receptor-mediated signaling pathways in regulating the actin cytoskeletal organization and cell migration (17–19). Overexpression of the HAS genes enhances the motility of a human melanoma cell line (20), and antisense suppression of HAS2 gene expression reduced keratinocyte migration (21). Important roles for the Ras signaling pathway in HA-dependent cell migration have been demonstrated by disruption of the HAS2 gene (22), and our recent study further indicated that simultaneous activation of Ras-mitogen-activated protein (MAP) kinase and PI3-kinase pathways is required for the activation of HA-dependent cell locomotion (16). CD44-HA interaction also triggers small GTP-binding protein signaling cascades, resulting in the rearrangement of the actin cytoskeleton and rapid outgrowth of lamellipodia (18, 19). Therefore, receptor-mediated signaling pathways are likely to cooperatively enhance the migration of the HAS transfectants into the wound leaving space.
Administration of soluble HA in the culture medium has been shown to activate intracellular mitogenic signals through HA-receptor interactions (23, 24). Here, we confirmed the effects of soluble HA on the enhancement of cell proliferation. However, the formation of stratified cell layers was not induced by administering exogenous soluble HA or the conditioned medium from HAS2 transfectants, suggesting that the massive HA matrix serves as a scaffold for the stratification of the cell layers. Overproduction of HA and an abnormal accumulation of matrix altered the distribution of N-cadherin at the intercellular boundaries and induced cell cycle progression in confluent cells. Because cadherin-mediated cell adhesion can regulate several components of the cell cycle machinery (14, 25, 26), our results led us to believe that the disruption of cell–cell adhesion caused by the abnormal accumulation of HA matrix accounts for the reduction of contact inhibition. A possible mechanism may be that the cell–cell adhesion was regulated via other components of the HA matrix, such as large chondroitin sulfate proteoglycans (27). Although the mechanisms that control the functional aspects of these phenotypes are not yet fully understood, the results of the present study suggested that the PI3-kinase pathway is also involved in regulating the stabilization of cell–cell adhesion and the contact inhibition of cell growth.
Uncontrolled production of HA and an abnormal accumulation of matrix led to a diminution of contact inhibition in a nontransformed, immortal cell line, which is an early event in cellular transformation. Overexpression of HAS2 does enhance the anchorage-independent growth and tumorigenicity of human fibrosarcoma cells (12), and overexpression of HAS1 enhances the metastatic ability of mouse mammary carcinoma cells (13). However, HAS-expressing 3Y1 cells did not show an enhancement of anchorage-independent growth or s.c. tumor formation. This result suggests that HA itself has no effect on the oncogenic transformation. The abnormal accumulation of matrix is therefore likely to enhance tumor progression when combined with other genetic alterations. Camenisch et al. demonstrated that Ras signaling activated by administering soluble HA is involved in the transformation of endothelial cells to mesenchyme (22). Based on our results, however, alterations of cell shape and cytoskeletal organization and formation of overlapping cell layers, all of which are characteristics of cellular transformation, are not induced in the 3Y1 cells by administering exogenous soluble HA, suggesting that another mechanism involving the accumulation of matrix is required for the cellular transformation. Therefore, it will be of great interest to elucidate roles of HA matrix at the different stages of tumor progression.
Acknowledgments
We thank Drs. Andrew P. Spicer (University of California at Davis) and John A. McDonald (Mayo Clinic Scottsdale) for providing mouse HAS2 cDNA. We thank Dr. Youji Ohnuki (Seikagaku Corp., Japan) for providing HA fragments. This work was supported in part by grants from Japan's Ministry of Education, Culture and Science, special coordination funds from the Science and Technology Agency of the Japanese Government, a grant from the Aichi Cancer Research Foundation, and a special research fund from Seikagaku Corporation.
Abbreviations
- HA
hyaluronan
- HAS
HA synthase
- GlcA
glucuronic acid
Note
While we were in the final stages of writing, an article was published that contributes interesting information to the topic of this paper (28).
References
- 1.Laurent T C, Fraser J R E. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 2.Knudson C B, Knudson W. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- 3.Weigel P H, Hascall V, Tammi M. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 4.Itano N, Kimata K. Trend Glycosci Glycotechnol. 1998;10:23–38. [Google Scholar]
- 5.Spicer A P, McDonald J A. J Biol Chem. 1998;273:1923–1932. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- 6.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, et al. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 7.Ishimoto N, Temin H M, Strominger J L. J Biol Chem. 1966;241:2052–2057. [PubMed] [Google Scholar]
- 8.Hopwood J J, Dorfman A. J Biol Chem. 1977;252:4777–4785. [PubMed] [Google Scholar]
- 9.Kimata K, Honma Y, Okayama M, Oguri K, Hozumi M, Suzuki S. Cancer Res. 1983;43:1347–1354. [PubMed] [Google Scholar]
- 10.Turley E A, Tretiak M. Cancer Res. 1985;45:5098–5105. [PubMed] [Google Scholar]
- 11.Simpson M A, Reiland J, Burger S R, Furcht L T, Spicer A P, Oegema T R, McCarthy J B. J Biol Chem. 2001;276:17949–17957. doi: 10.1074/jbc.M010064200. [DOI] [PubMed] [Google Scholar]
- 12.Kosaki R, Watanabe K, Yamaguchi Y. Cancer Res. 1999;59:1141–1145. [PubMed] [Google Scholar]
- 13.Itano N, Sawai T, Miyaishi O, Kimata K. Cancer Res. 1999;59:2499–2504. [PubMed] [Google Scholar]
- 14.Croix B S, Sheehan C, Rak J W, Florenes V A, Slingerland J M, Kerbel R S. J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura G, Itagaki A, Summers J. Int J Cancer. 1975;15:694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- 16.Sohara Y, Iahiguro N, Machida K, Kurata H, Thant A A, Senga T, Matsuda S, Kimata K, Iwata H, Hamaguchi M. Mol Biol Cell. 2001;12:1859–1868. doi: 10.1091/mbc.12.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Entwistle J, Hall C L, Turley E A. J Cell Biochem. 1996;61:569–577. doi: 10.1002/(sici)1097-4644(19960616)61:4<569::aid-jcb10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Oliferenko S, Kaverina I, Small V, Huber L A. J Cell Biol. 2000;148:1159–1164. doi: 10.1083/jcb.148.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourguignon L Y, Zhu H, Shao L, Chen Y W. J Biol Chem. 2000;275:1829–1838. doi: 10.1074/jbc.275.3.1829. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa T, Itano N, Sawai T, Kimata K, Koganehira Y, Saida T, Taniguchi S. J Invest Dermatol. 1999;113:935–939. doi: 10.1046/j.1523-1747.1999.00804.x. [DOI] [PubMed] [Google Scholar]
- 21.Pienimaki J P, Rilla K, Fulop C, Sironen R K, Karvinen S, Pasonen S, Lammi M J, Tammi R, Hascall V C, Tammi M I. J Biol Chem. 2001;276:20428–20435. doi: 10.1074/jbc.M007601200. [DOI] [PubMed] [Google Scholar]
- 22.Camenisch T D, Spicer A P, Brehm-Gibson T, Biesterfeldt J, Augustine M L, Calabro A, Jr, Kubalak S, Klewer S E, McDonald J A. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slevin M, Krupinski J, Kumar S, Gaffney J. Lab Invest. 1998;78:987–1003. [PubMed] [Google Scholar]
- 24.Serbulea M, Kakumu S, Thant A A, Miyazaki K, Machida K, Senga T, Ohta S, Yoshioka K, Hotta N, Hamaguchi M. Int J Oncol. 1999;14:733–738. doi: 10.3892/ijo.14.4.733. [DOI] [PubMed] [Google Scholar]
- 25.Levenberg S, Yarden A, Kam Z, Geiger B. Oncogene. 1998;18:869–876. doi: 10.1038/sj.onc.1202396. [DOI] [PubMed] [Google Scholar]
- 26.Orford K, Orford C C, Byers S W. J Cell Biol. 1999;146:855–867. doi: 10.1083/jcb.146.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vleminckx K L, Deman J J, Bruyneel E A, Vandenbossche G M, Keirsebilck A A, Mareel M M, van Roy F M. Cancer Res. 1994;54:873–877. [PubMed] [Google Scholar]
- 28.Liu N, Gao F, Han Z, Xu X, Underhill C B, Zhang L. Cancer Res. 2001;61:5207–5214. [PubMed] [Google Scholar]