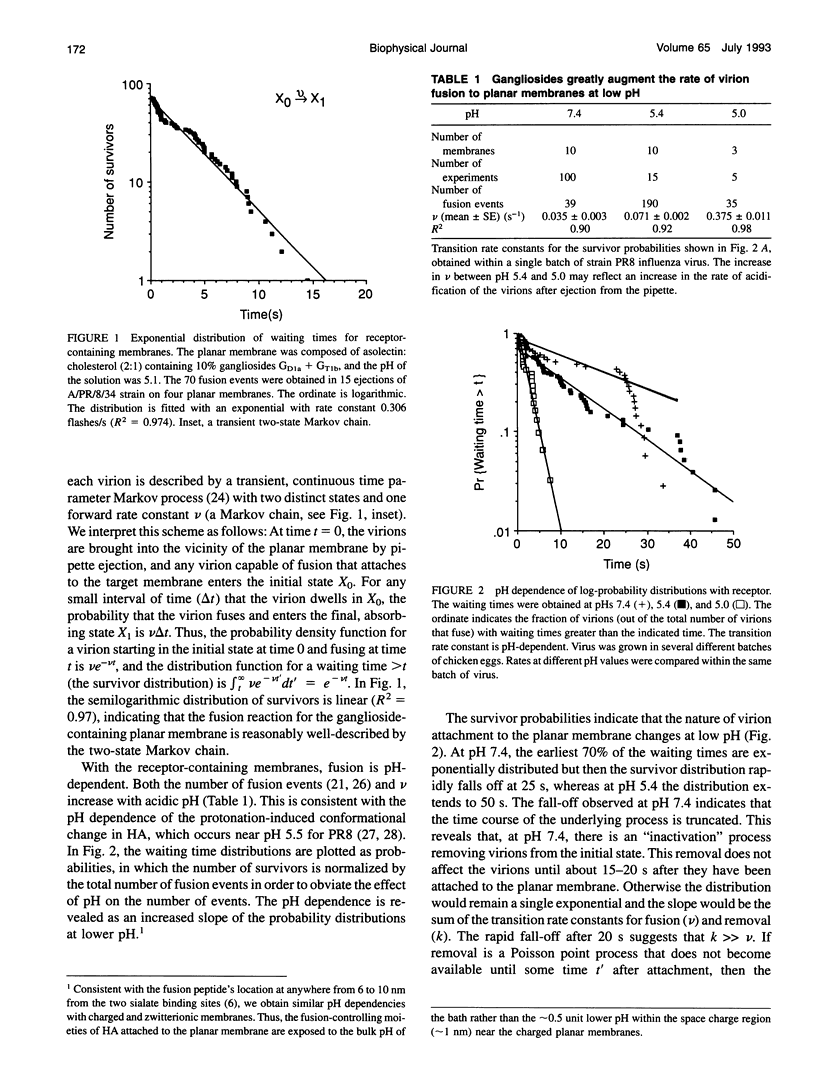
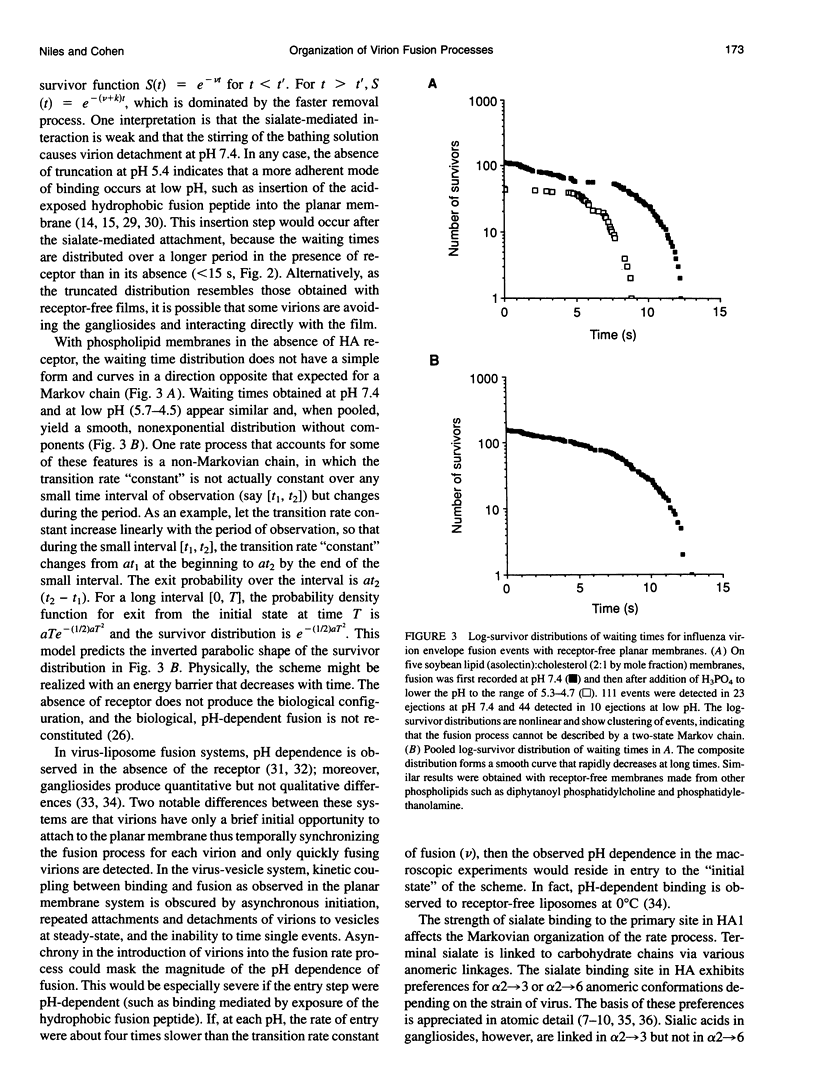
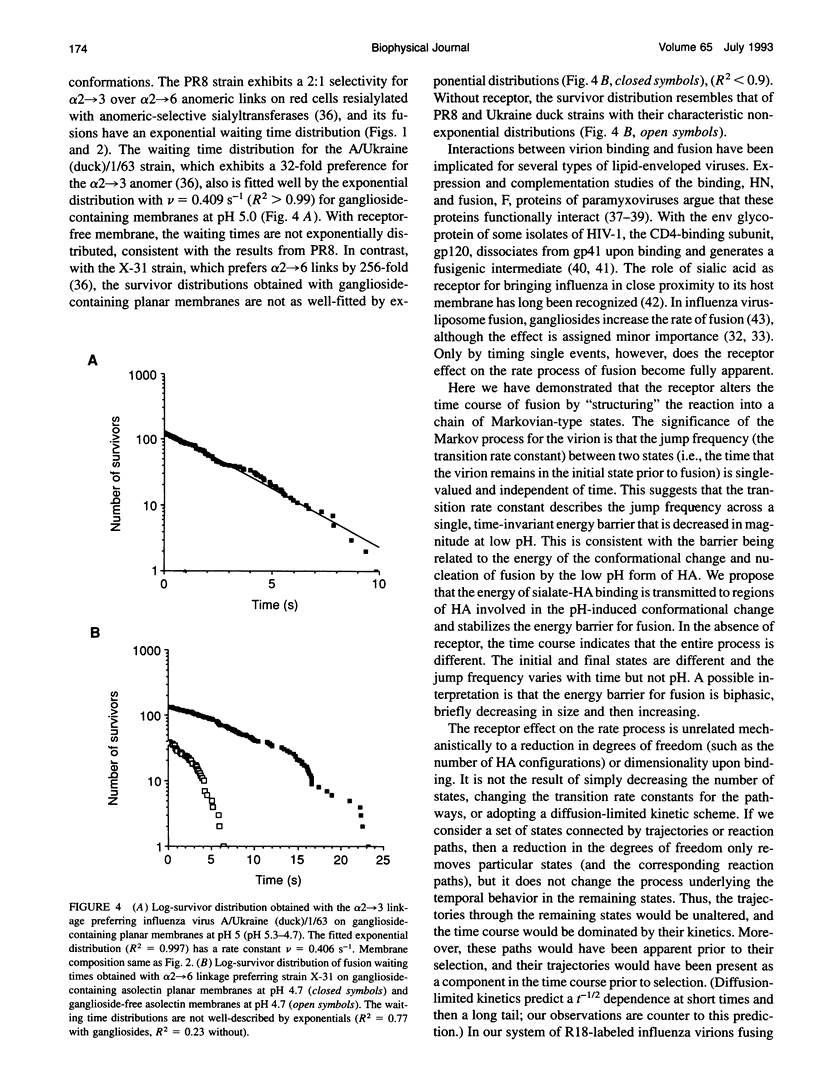
Abstract
The initial steps of membrane fusion, receptor binding and membrane destabilization, are mediated by the envelope glycoprotein hemagglutinin of influenza virus. Interaction between these functions was determined from the time course of individual virion fusions to a planar membrane with and without receptor. With receptor, fusion was described by a Poisson process. In the absence of receptor, the time course was more complicated and could not be described with exponential rate constants. The conversion of a non-Markovian process into a simple Markov chain is direct evidence that receptor binding fundamentally alters the route of fusion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S. Receptor-mediated activation of immunodeficiency viruses in viral fusion. Science. 1991 May 31;252(5010):1322–1323. doi: 10.1126/science.1925547. [DOI] [PubMed] [Google Scholar]
- Brunner J., Zugliani C., Mischler R. Fusion activity of influenza virus PR8/34 correlates with a temperature-induced conformational change within the hemagglutinin ectodomain detected by photochemical labeling. Biochemistry. 1991 Mar 5;30(9):2432–2438. doi: 10.1021/bi00223a019. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Helenius A., White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985 Mar 10;260(5):2973–2981. [PubMed] [Google Scholar]
- Ebata S. N., Côté M. J., Kang C. Y., Dimock K. The fusion and hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus 3 are both required for fusion. Virology. 1991 Jul;183(1):437–441. doi: 10.1016/0042-6822(91)90162-5. [DOI] [PubMed] [Google Scholar]
- Godley L., Pfeifer J., Steinhauer D., Ely B., Shaw G., Kaufmann R., Suchanek E., Pabo C., Skehel J. J., Wiley D. C. Introduction of intersubunit disulfide bonds in the membrane-distal region of the influenza hemagglutinin abolishes membrane fusion activity. Cell. 1992 Feb 21;68(4):635–645. doi: 10.1016/0092-8674(92)90140-8. [DOI] [PubMed] [Google Scholar]
- HIRST G. K. The nature of the virus receptors of red cells; evidence on the chemical nature of the virus receptors of red cells and of the existence of a closely analogous substance in normal serum. J Exp Med. 1948 Apr 1;87(4):301–314. doi: 10.1084/jem.87.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., de Boer T., Klappe K., Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984 Nov 20;23(24):5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Hu X. L., Ray R., Compans R. W. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J Virol. 1992 Mar;66(3):1528–1534. doi: 10.1128/jvi.66.3.1528-1534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. T., Rott R., Klenk H. D. Influenza viruses cause hemolysis and fusion of cells. Virology. 1981 Apr 15;110(1):243–247. doi: 10.1016/0042-6822(81)90030-1. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Kemble G. W., Bodian D. L., Rosé J., Wilson I. A., White J. M. Intermonomer disulfide bonds impair the fusion activity of influenza virus hemagglutinin. J Virol. 1992 Aug;66(8):4940–4950. doi: 10.1128/jvi.66.8.4940-4950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Morrison T., McQuain C., McGinnes L. Complementation between avirulent Newcastle disease virus and a fusion protein gene expressed from a retrovirus vector: requirements for membrane fusion. J Virol. 1991 Feb;65(2):813–822. doi: 10.1128/jvi.65.2.813-822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles W. D., Cohen F. S. Fusion of influenza virions with a planar lipid membrane detected by video fluorescence microscopy. J Gen Physiol. 1991 Jun;97(6):1101–1119. doi: 10.1085/jgp.97.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles W. D., Cohen F. S. The role of N-acetylneuraminic (sialic) acid in the pH dependence of influenza virion fusion with planar phospholipid membranes. J Gen Physiol. 1991 Jun;97(6):1121–1140. doi: 10.1085/jgp.97.6.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles W. D., Cohen F. S. Video fluorescence microscopy studies of phospholipid vesicle fusion with a planar phospholipid membrane. Nature of membrane-membrane interactions and detection of release of contents. J Gen Physiol. 1987 Nov;90(5):703–735. doi: 10.1085/jgp.90.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles W. D., Li Q., Cohen F. S. Computer detection of the rapid diffusion of fluorescent membrane fusion markers in images observed with video microscopy. Biophys J. 1992 Sep;63(3):710–722. doi: 10.1016/S0006-3495(92)81641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983 Jul 7;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983 Jun;127(2):361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Sauter N. K., Bednarski M. D., Wurzburg B. A., Hanson J. E., Whitesides G. M., Skehel J. J., Wiley D. C. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry. 1989 Oct 17;28(21):8388–8396. doi: 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- Sauter N. K., Glick G. D., Crowther R. L., Park S. J., Eisen M. B., Skehel J. J., Knowles J. R., Wiley D. C. Crystallographic detection of a second ligand binding site in influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):324–328. doi: 10.1073/pnas.89.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T., Delfino J. M., Richards F. M., Helenius A. The HA2 subunit of influenza hemagglutinin inserts into the target membrane prior to fusion. J Biol Chem. 1991 Sep 25;266(27):18404–18410. [PubMed] [Google Scholar]
- Stegmann T., Nir S., Wilschut J. Membrane fusion activity of influenza virus. Effects of gangliosides and negatively charged phospholipids in target liposomes. Biochemistry. 1989 Feb 21;28(4):1698–1704. doi: 10.1021/bi00430a041. [DOI] [PubMed] [Google Scholar]
- Stegmann T., White J. M., Helenius A. Intermediates in influenza induced membrane fusion. EMBO J. 1990 Dec;9(13):4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Nagao Y., Kato H., Matsumoto M., Nerome K., Nakajima K., Nobusawa E. Human influenza A virus hemagglutinin distinguishes sialyloligosaccharides in membrane-associated gangliosides as its receptor which mediates the adsorption and fusion processes of virus infection. Specificity for oligosaccharides and sialic acids and the sequence to which sialic acid is attached. J Biol Chem. 1986 Dec 25;261(36):17057–17061. [PubMed] [Google Scholar]
- Südhof T. C., Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991 May;6(5):665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Linial M., Scheller R. H. Cellular and molecular biology of the presynaptic nerve terminal. Annu Rev Neurosci. 1991;14:93–122. doi: 10.1146/annurev.ne.14.030191.000521. [DOI] [PubMed] [Google Scholar]
- Vogel S. S., Zimmerberg J. Proteins on exocytic vesicles mediate calcium-triggered fusion. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4749–4753. doi: 10.1073/pnas.89.10.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Wharton S. A., Skehel J. J., Wiley D. C. Studies of influenza haemagglutinin-mediated membrane fusion. Virology. 1986 Feb;149(1):27–35. doi: 10.1016/0042-6822(86)90083-8. [DOI] [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- White J. M., Wilson I. A. Anti-peptide antibodies detect steps in a protein conformational change: low-pH activation of the influenza virus hemagglutinin. J Cell Biol. 1987 Dec;105(6 Pt 2):2887–2896. doi: 10.1083/jcb.105.6.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1(2):217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Wunderli-Allenspach H., Ott S. Kinetics of fusion and lipid transfer between virus receptor containing liposomes and influenza viruses as measured with the octadecylrhodamine B chloride assay. Biochemistry. 1990 Feb 27;29(8):1990–1997. doi: 10.1021/bi00460a005. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Gerhard W., Bachi T. Monoclonal anti-hemagglutinin antibodies detect irreversible antigenic alterations that coincide with the acid activation of influenza virus A/PR/834-mediated hemolysis. J Virol. 1983 Oct;48(1):239–248. doi: 10.1128/jvi.48.1.239-248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Kuroda K., Kawasaki K., Yamashina S., Maeda T., Ohnishi S. Infectious cell entry mechanism of influenza virus. J Virol. 1982 Jul;43(1):284–293. doi: 10.1128/jvi.43.1.284-293.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


