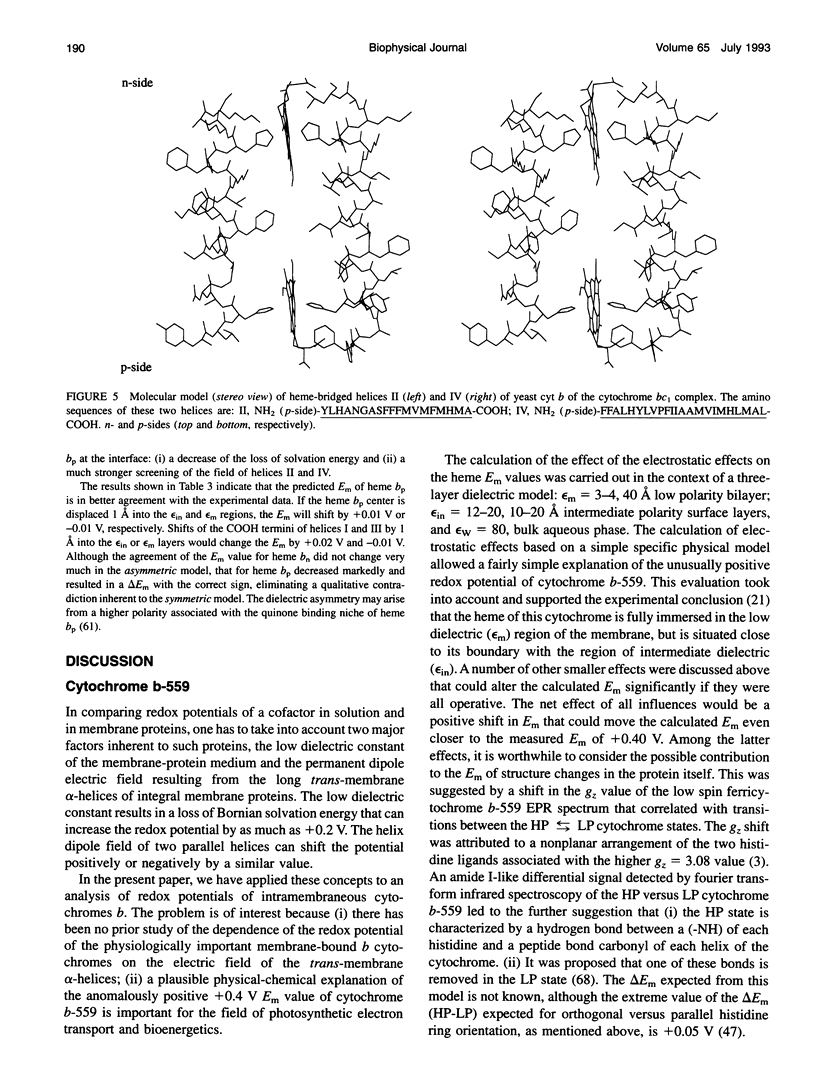
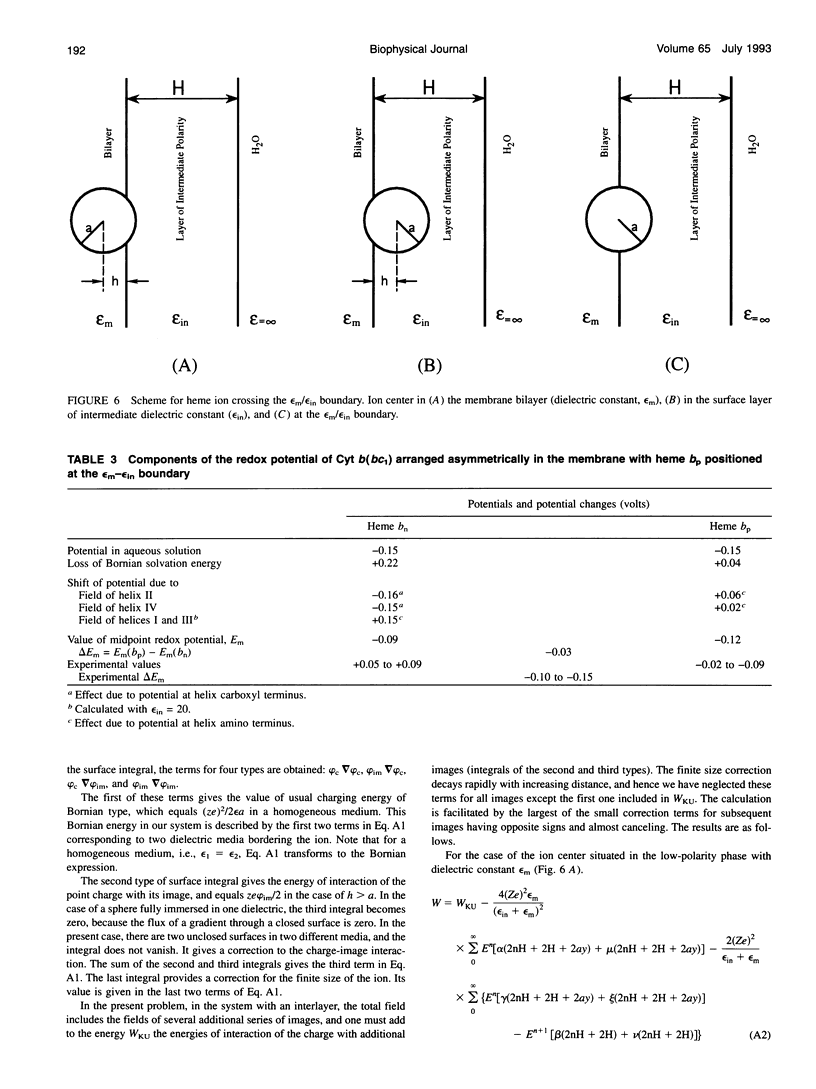
Abstract
The effect of the dipole potential field of extended membrane spanning alpha-helices on the redox potentials of b cytochromes in energy transducing membranes has been calculated in the context of a three phase model for the membrane. In this model, the membrane contains three dielectric layers; (i) a 40-A hydrophobic membrane bilayer, with dielectric constant em = 3-4, (ii) 10-20-A interfacial layers of intermediate polarity, ein = 12-20, that consist of lipid polar head groups and peripheral protein segments, and (iii) an external infinite water medium, ew = 80. The unusually positive midpoint potential, Em = +0.4 V, of the "high potential" cytochrome b-559 of oxygenic photosynthetic membranes, a previously enigmatic property of this cytochrome, can be explained by (i) the position of the heme in the positive dipole potential region near the NH2 termini of the two parallel helices that provide its histidine ligands, and (ii) the loss of solvation energy of the heme ion due to the low dielectric constant of its surroundings, leading to an estimate of +0.31 to +0.37 V for the cytochrome Em. The known tendency of this cytochrome to undergo a large -delta Em shift upon exposure of thylakoid membranes to proteases or damaging treatments is explained by disruption of the intermediate polarity (ein) surface dielectric layer and the resulting contact of the heme with the external water medium. Application of this model to the two hemes (bn and bp) of cytochrome b of the cytochrome bc1 complex, with the two hemes placed symmetrically in the low dielectric (em) membrane bilayer, results in Em values of hemes bn and bp that are, respectively, somewhat too negative (approximately -0.1 V), and much too positive (approximately +0.3 V), leading to a potential difference, Em(bp) - Em(bn), with the wrong sign and magnitude, +0.25 V instead of -0.10 to -0.15 V. The heme potentials can only be approximately reconciled with experiment, if it is assumed that the two hemes are in different dielectric environments, with that of heme bp being more polar.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Widger W. R., Cramer W. A., Oertling W. A., Metz J. G. Axial ligands of chloroplast cytochrome b-559: identification and requirement for a heme-cross-linked polypeptide structure. Biochemistry. 1985 Jul 2;24(14):3638–3645. doi: 10.1021/bi00335a036. [DOI] [PubMed] [Google Scholar]
- Berthomieu C., Boussac A., Mäntele W., Breton J., Nabedryk E. Molecular changes following oxidoreduction of cytochrome b559 characterized by Fourier transform infrared difference spectroscopy and electron paramagnetic resonance: photooxidation in photosystem II and electrochemistry of isolated cytochrome b559 and iron protoporphyrin IX-bisimidazole model compounds. Biochemistry. 1992 Nov 24;31(46):11460–11471. doi: 10.1021/bi00161a026. [DOI] [PubMed] [Google Scholar]
- Brasseur R. Calculation of the three-dimensional structure of Saccharomyces cerevisiae cytochrome b inserted in a lipid matrix. J Biol Chem. 1988 Sep 5;263(25):12571–12575. [PubMed] [Google Scholar]
- Cevc G., Watts A., Marsh D. Titration of the phase transition of phosphatidylserine bilayer membranes. Effects of pH, surface electrostatics, ion binding, and head-group hydration. Biochemistry. 1981 Aug 18;20(17):4955–4965. doi: 10.1021/bi00520a023. [DOI] [PubMed] [Google Scholar]
- Cramer W. A., Whitmarsh J., Low P. S. Differential scanning calorimetry of chloroplast membranes: identification of an endothermic transition associated with the water-splitting complex of photosystem II. Biochemistry. 1981 Jan 6;20(1):157–162. doi: 10.1021/bi00504a026. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. Nobel lecture. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989 Aug;8(8):2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposti M. D. Prediction and comparison of the haem-binding sites in membrane haemoproteins. Biochim Biophys Acta. 1989 Dec 7;977(3):249–265. doi: 10.1016/s0005-2728(89)80079-9. [DOI] [PubMed] [Google Scholar]
- Fan H. N., Cramer W. A. The redox poential of cytochromes b-559 and b-563 in spinach chloroplasts. Biochim Biophys Acta. 1970 Aug 4;216(1):200–207. doi: 10.1016/0005-2728(70)90171-4. [DOI] [PubMed] [Google Scholar]
- Glaser E. G., Crofts A. R. A new electrogenic step in the ubiquinol:cytochrome c2 oxidoreductase complex of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1984 Aug 31;766(2):322–333. doi: 10.1016/0005-2728(84)90248-2. [DOI] [PubMed] [Google Scholar]
- Gunner M. R., Honig B. Electrostatic control of midpoint potentials in the cytochrome subunit of the Rhodopseudomonas viridis reaction center. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9151–9155. doi: 10.1073/pnas.88.20.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol W. G. The role of the alpha-helix dipole in protein function and structure. Prog Biophys Mol Biol. 1985;45(3):149–195. doi: 10.1016/0079-6107(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Horton P., Whitmarsh J., Cramer W. A. On the specific site of action of 3-)3,4-dichlorophenyl)-1,1-dimethylurea in chloroplasts: inhibiion of a dark acid-induced decrease in midpoint potential of cytochrome b-559. Arch Biochem Biophys. 1976 Oct;176(2):519–524. doi: 10.1016/0003-9861(76)90195-8. [DOI] [PubMed] [Google Scholar]
- Kassner R. J. Effects of nonpolar environments on the redox potentials of heme complexes. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2263–2267. doi: 10.1073/pnas.69.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtalik L. I. Effective activation energy of enzymatic and nonenzymatic reactions. Evolution-imposed requirements to enzyme structure. J Theor Biol. 1985 Jan 21;112(2):251–264. doi: 10.1016/s0022-5193(85)80285-x. [DOI] [PubMed] [Google Scholar]
- Krishtalik L. I., Topolev V. V. Vnutriglobuliarnoe elektrostaticheskoe pole fermenta. I. Pervichnoe pole, sozdavaemoe polipeptidnym ostovom, funktsional'nymi gruppami i ionami molekuly al'fa-khimotripsina. Mol Biol (Mosk) 1983 Sep-Oct;17(5):1034–1041. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lelkes P. I., Miller I. R. Perturbations of membrane structure by optical probes: I. Location and structural sensitivity of merocyanine 540 bound to phospholipid membranes. J Membr Biol. 1980 Jan 31;52(1):1–15. doi: 10.1007/BF01869001. [DOI] [PubMed] [Google Scholar]
- Moore G. R. Control of redox properties of cytochrome c by special electrostatic interactions. FEBS Lett. 1983 Sep 19;161(2):171–175. doi: 10.1016/0014-5793(83)81001-1. [DOI] [PubMed] [Google Scholar]
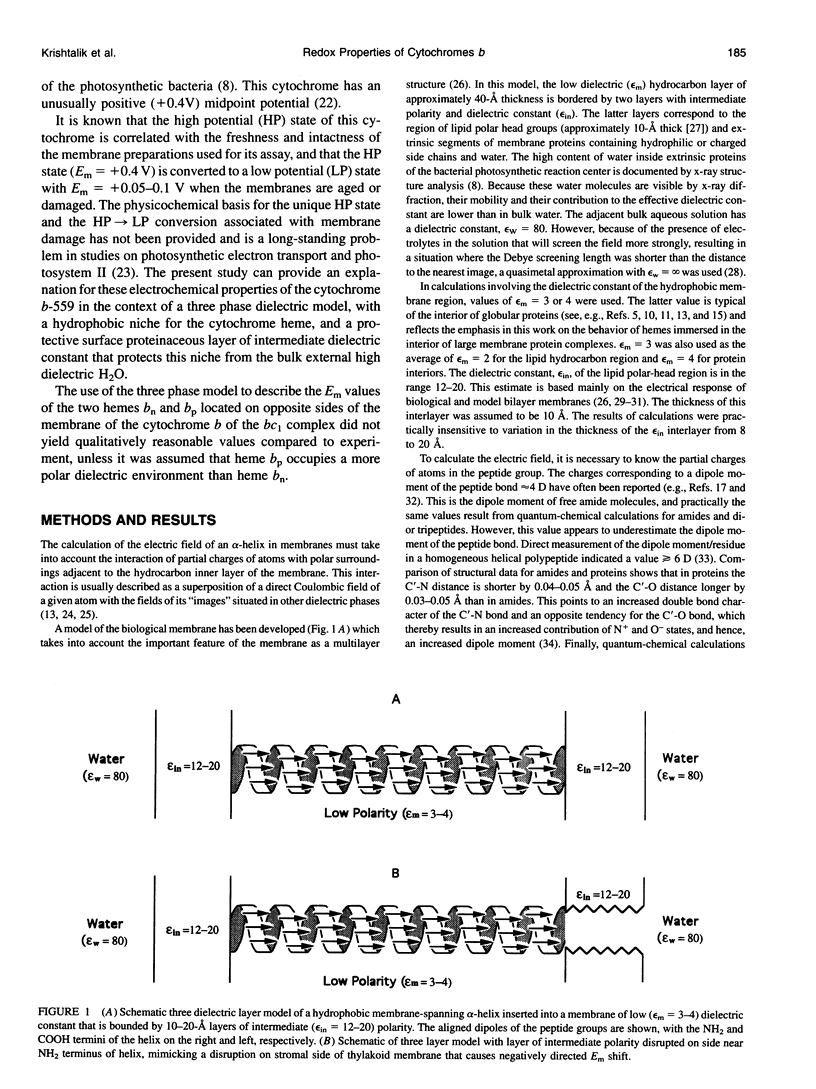
- Moore G. R., Pettigrew G. W., Rogers N. K. Factors influencing redox potentials of electron transfer proteins. Proc Natl Acad Sci U S A. 1986 Jul;83(14):4998–4999. doi: 10.1073/pnas.83.14.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Läuger P. Nonlinear electrical effects in lipid bilayer membranes. II. Integration of the generalized Nernst-Planck equations. Biophys J. 1969 Sep;9(9):1160–1170. doi: 10.1016/S0006-3495(69)86443-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Ohnishi T., Schägger H., Meinhardt S. W., LoBrutto R., Link T. A., von Jagow G. Spatial organization of redox active centers in the bovine heart ubiquinol-cytochrome c oxidoreductase. J Biol Chem. 1989 Jan 15;264(2):735–744. [PubMed] [Google Scholar]
- Rich P. R., Jeal A. E., Madgwick S. A., Moody A. J. Inhibitor effects on redox-linked protonations of the b haems of the mitochondrial bc1 complex. Biochim Biophys Acta. 1990 Jul 17;1018(1):29–40. doi: 10.1016/0005-2728(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Daldal F., Dutton P. L. Mutants of ubiquinol-cytochrome c2 oxidoreductase resistant to Qo site inhibitors: consequences for ubiquinone and ubiquinol affinity and catalysis. Biochemistry. 1990 Dec 25;29(51):11249–11260. doi: 10.1021/bi00503a014. [DOI] [PubMed] [Google Scholar]
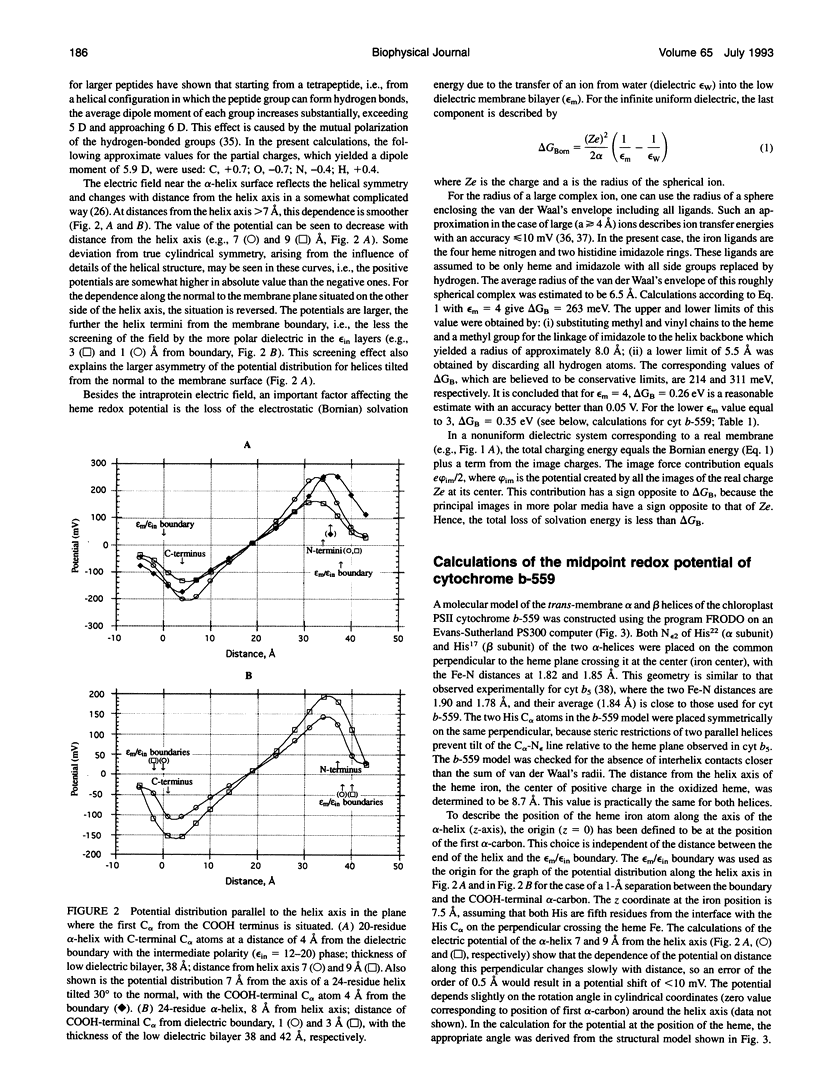
- Robertson D. E., Dutton P. L. The nature and magnitude of the charge-separation reactions of ubiquinol cytochrome c2 oxidoreductase. Biochim Biophys Acta. 1988 Oct 5;935(3):273–291. doi: 10.1016/0005-2728(88)90223-x. [DOI] [PubMed] [Google Scholar]
- Rogers N. K., Moore G. R., Sternberg M. J. Electrostatic interactions in globular proteins: calculation of the pH dependence of the redox potential of cytochrome c551. J Mol Biol. 1985 Apr 20;182(4):613–616. doi: 10.1016/0022-2836(85)90248-7. [DOI] [PubMed] [Google Scholar]
- Rogers N. K. The modelling of electrostatic interactions in the function of globular proteins. Prog Biophys Mol Biol. 1986;48(1):37–66. doi: 10.1016/0079-6107(86)90009-x. [DOI] [PubMed] [Google Scholar]
- Saraste M. Location of haem-binding sites in the mitochondrial cytochrome b. FEBS Lett. 1984 Jan 30;166(2):367–372. doi: 10.1016/0014-5793(84)80114-3. [DOI] [PubMed] [Google Scholar]
- Sharp K. A., Honig B. Electrostatic interactions in macromolecules: theory and applications. Annu Rev Biophys Biophys Chem. 1990;19:301–332. doi: 10.1146/annurev.bb.19.060190.001505. [DOI] [PubMed] [Google Scholar]
- Sheridan R. P., Allen L. C. The electrostatic potential of the alpha helix (electrostatic potential/alpha-helix/secondary structure/helix dipole). Biophys Chem. 1980 Apr;11(2):133–136. doi: 10.1016/0301-4622(80)80015-9. [DOI] [PubMed] [Google Scholar]
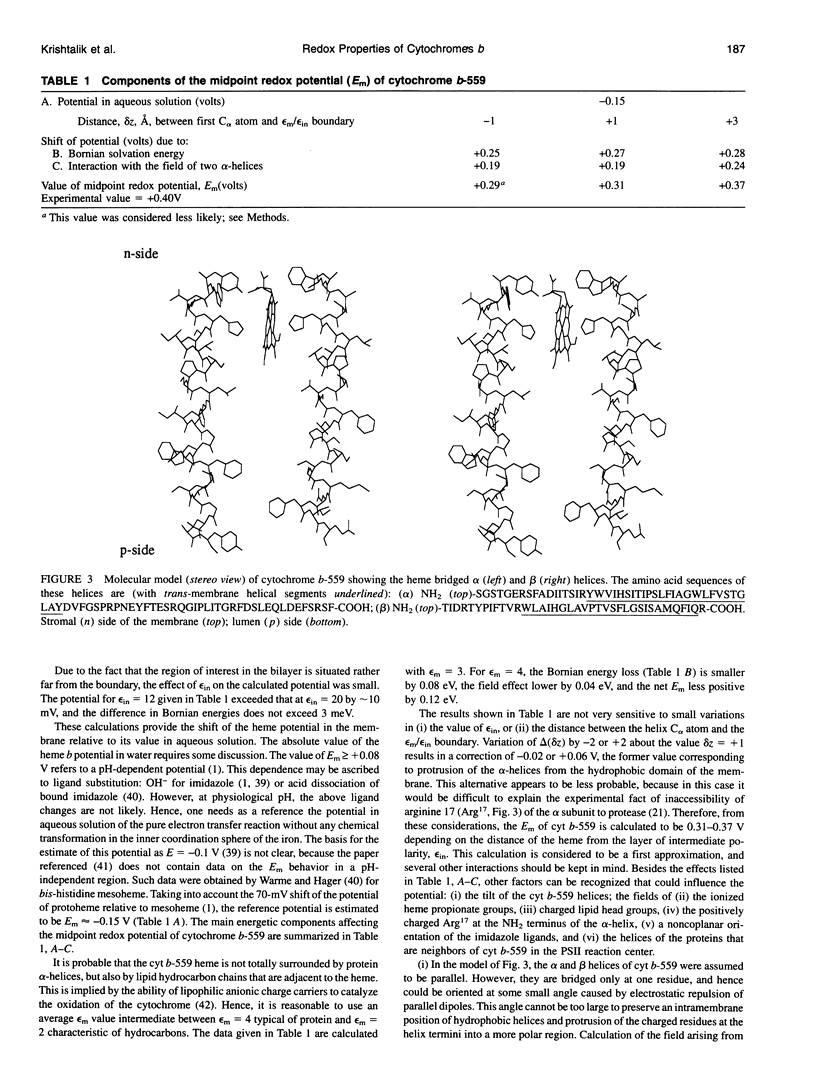
- Szczepaniak A., Cramer W. A. Thylakoid membrane protein topography. Location of the termini of the chloroplast cytochrome b6 on the stromal side of the membrane. J Biol Chem. 1990 Oct 15;265(29):17720–17726. [PubMed] [Google Scholar]
- Tae G. S., Black M. T., Cramer W. A., Vallon O., Bogorad L. Thylakoid membrane protein topography: transmembrane orientation of the chloroplast cytochrome b-559 psbE gene product. Biochemistry. 1988 Dec 27;27(26):9075–9080. doi: 10.1021/bi00426a002. [DOI] [PubMed] [Google Scholar]
- Tang X. S., Fushimi K., Satoh K. D1-D2 complex of the photosystem II reaction center from spinach. Isolation and partial characterization. FEBS Lett. 1990 Oct 29;273(1-2):257–260. doi: 10.1016/0014-5793(90)81098-9. [DOI] [PubMed] [Google Scholar]
- Warme P. K., Hager L. P. Heme sulfuric anhydrides. II. Properties of heme models prepared from mesoheme sulfuric anhydrides. Biochemistry. 1970 Mar 31;9(7):1606–1614. doi: 10.1021/bi00809a020. [DOI] [PubMed] [Google Scholar]
- Warshel A., Aqvist J. Electrostatic energy and macromolecular function. Annu Rev Biophys Biophys Chem. 1991;20:267–298. doi: 10.1146/annurev.bb.20.060191.001411. [DOI] [PubMed] [Google Scholar]
- Warshel A., Russell S. T. Calculations of electrostatic interactions in biological systems and in solutions. Q Rev Biophys. 1984 Aug;17(3):283–422. doi: 10.1017/s0033583500005333. [DOI] [PubMed] [Google Scholar]
- Whitmarsh J., Cramer W. A. Kinetics of the photoreduction of cytochrome b-559 by photosystem II in chloroplasts. Biochim Biophys Acta. 1977 May 11;460(2):280–289. doi: 10.1016/0005-2728(77)90214-6. [DOI] [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C. H., Crofts A. R., Gennis R. B. Assignment of the histidine axial ligands to the cytochrome bH and cytochrome bL components of the bc1 complex from Rhodobacter sphaeroides by site-directed mutagenesis. Biochemistry. 1991 Jul 9;30(27):6747–6754. doi: 10.1021/bi00241a017. [DOI] [PubMed] [Google Scholar]
- Yun C. H., Van Doren S. R., Crofts A. R., Gennis R. B. The use of gene fusions to examine the membrane topology of the L-subunit of the photosynthetic reaction center and of the cytochrome b subunit of the bc1 complex from Rhodobacter sphaeroides. J Biol Chem. 1991 Jun 15;266(17):10967–10973. [PubMed] [Google Scholar]
- di Rago J. P., Colson A. M. Molecular basis for resistance to antimycin and diuron, Q-cycle inhibitors acting at the Qi site in the mitochondrial ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. J Biol Chem. 1988 Sep 5;263(25):12564–12570. [PubMed] [Google Scholar]
- di Rago J. P., Coppée J. Y., Colson A. M. Molecular basis for resistance to myxothiazol, mucidin (strobilurin A), and stigmatellin. Cytochrome b inhibitors acting at the center o of the mitochondrial ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. J Biol Chem. 1989 Aug 25;264(24):14543–14548. [PubMed] [Google Scholar]
- di Rago J. P., Netter P., Slonimski P. P. Intragenic suppressors reveal long distance interactions between inactivating and reactivating amino acid replacements generating three-dimensional constraints in the structure of mitochondrial cytochrome b. J Biol Chem. 1990 Sep 15;265(26):15750–15757. [PubMed] [Google Scholar]