Abstract
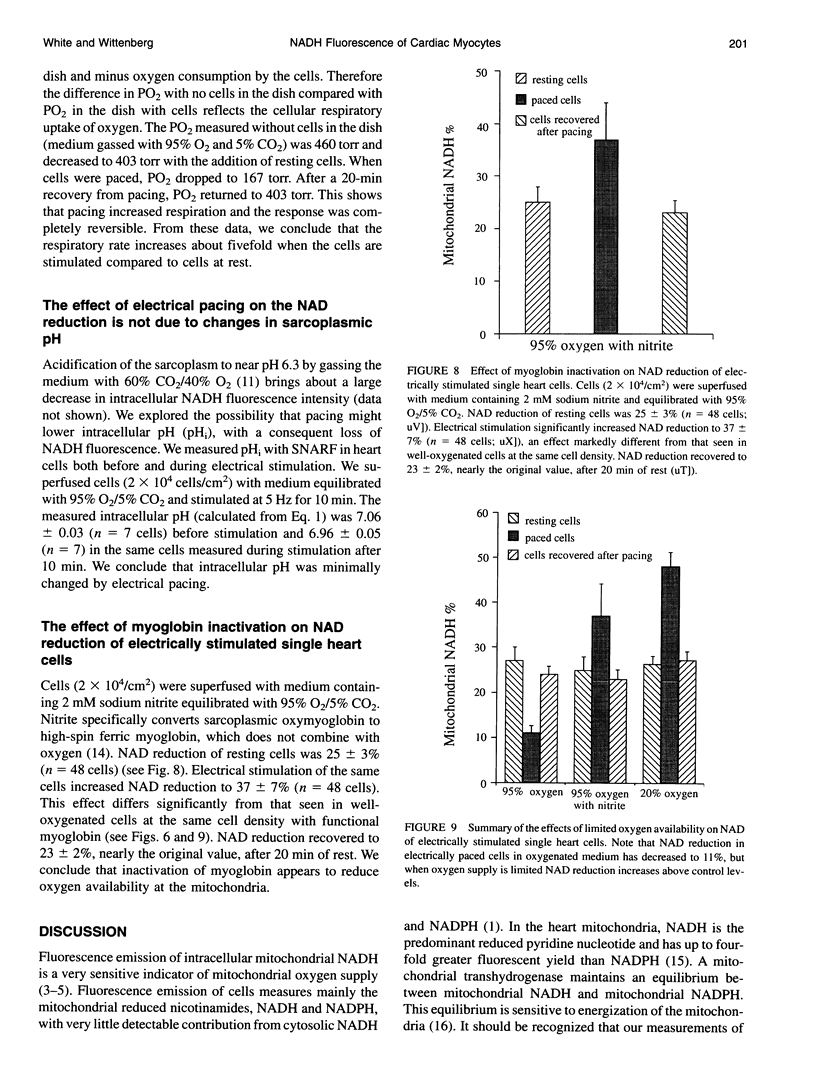
Endogenous fluorescence was used to measure the extent of reduction of mitochondrial NAD in individual, isolated rat cardiac myocytes. NAD reduction was determined from emitted fluorescence at 415 and 470 nm during brief epi-illumination at 365 nm. NAD reduction of resting myocytes, superfused with medium equilibrated with 95% O2/5% CO2, was 27 +/- 3% (SE) (n = 78), comparable to that in beating whole heart. Increasing intracellular Ca2+ did not significantly change NAD reduction. NAD reduction decreased reversibly to 11 +/- 1% (n = 78) in contracting myocytes electrically paced at 5 Hz for 10 min. Oxygen uptake was stimulated fivefold. There was minimal change in sarcoplasmic pH measured by fluorescence of carboxy-seminaphthorhodafluor-1. However, NAD reduction increased reversibly in response to electrically paced contractions when: (a) myoglobin was inactivated with sodium nitrite (37 +/- 7%; n = 48); or (b) cells were more densely layered and gassed with 20% O2/5% CO2 (48 +/- 3%; n = 30). We conclude that (a) the ratio NADH/NAD is decreased in well-oxygenated cells with increased work; (b) steady-state NAD reduction is increased with increased work when oxygen delivery is limited; and (c) functional myoglobin ensures an oxygen supply to the mitochondria of working cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkich D. A., Williams G. D., Masiakos P. T., Smith M. B., Boyer P. D., LaNoue K. F. Rates of various reactions catalyzed by ATP synthase as related to the mechanism of ATP synthesis. J Biol Chem. 1991 Jan 5;266(1):123–129. [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H., MacLeod K. T. The mechanism of ryanodine action in rabbit ventricular muscle evaluated with Ca-selective microelectrodes and rapid cooling contractures. Can J Physiol Pharmacol. 1987 Apr;65(4):610–618. doi: 10.1139/y87-103. [DOI] [PubMed] [Google Scholar]
- Blank P. S., Silverman H. S., Chung O. Y., Hogue B. A., Stern M. D., Hansford R. G., Lakatta E. G., Capogrossi M. C. Cytosolic pH measurements in single cardiac myocytes using carboxy-seminaphthorhodafluor-1. Am J Physiol. 1992 Jul;263(1 Pt 2):H276–H284. doi: 10.1152/ajpheart.1992.263.1.H276. [DOI] [PubMed] [Google Scholar]
- Chapman J. B. Fluorometric studies of oxidative metabolism in isolated papillary muscle of the rabbit. J Gen Physiol. 1972 Feb;59(2):135–154. doi: 10.1085/jgp.59.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller J. E., Wittenberg B. A. Intracellular calcium and high-energy phosphates in isolated cardiac myocytes. Am J Physiol. 1990 Dec;259(6 Pt 2):H1851–H1859. doi: 10.1152/ajpheart.1990.259.6.H1851. [DOI] [PubMed] [Google Scholar]
- Doeller J. E., Wittenberg B. A. Myoglobin function and energy metabolism of isolated cardiac myocytes: effect of sodium nitrite. Am J Physiol. 1991 Jul;261(1 Pt 2):H53–H62. doi: 10.1152/ajpheart.1991.261.1.H53. [DOI] [PubMed] [Google Scholar]
- Eng J., Lynch R. M., Balaban R. S. Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J. 1989 Apr;55(4):621–630. doi: 10.1016/S0006-3495(89)82859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi K., Nishida M., Shaw D., Smith T. W., Marsh J. D. NADH measurements in adult rat myocytes during simulated ischemia. Am J Physiol. 1991 Jun;260(6 Pt 2):H1743–H1752. doi: 10.1152/ajpheart.1991.260.6.H1743. [DOI] [PubMed] [Google Scholar]
- From A. H., Zimmer S. D., Michurski S. P., Mohanakrishnan P., Ulstad V. K., Thoma W. J., Uğurbil K. Regulation of the oxidative phosphorylation rate in the intact cell. Biochemistry. 1990 Apr 17;29(15):3731–3743. doi: 10.1021/bi00467a020. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Rydström J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J. 1988 Aug 15;254(1):1–10. doi: 10.1042/bj2540001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl C. M., Wimsatt D. K., Brierley G. P., Altschuld R. A. IMP production by ATP-depleted adult rat heart cells. Effects of glycolysis and alpha 1-adrenergic stimulation. Circ Res. 1989 Sep;65(3):754–760. doi: 10.1161/01.res.65.3.754. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F., Duffield J. C. Oxidative and glycolytic recovery metabolism in muscle. J Gen Physiol. 1967 Mar;50(4):1009–1047. doi: 10.1085/jgp.50.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. A., Koretsky A. P., Balaban R. S. Respiratory control in the glucose perfused heart. A 31P NMR and NADH fluorescence study. FEBS Lett. 1987 Sep 14;221(2):270–276. doi: 10.1016/0014-5793(87)80939-0. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Halestrap A. P., Denton R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990 Apr;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Miyata H., Silverman H. S., Sollott S. J., Lakatta E. G., Stern M. D., Hansford R. G. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991 Oct;261(4 Pt 2):H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- Moreno-Sánchez R., Hansford R. G. Relation between cytosolic free calcium and respiratory rates in cardiac myocytes. Am J Physiol. 1988 Aug;255(2 Pt 2):H347–H357. doi: 10.1152/ajpheart.1988.255.2.H347. [DOI] [PubMed] [Google Scholar]
- Nuutinen E. M. Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart. Basic Res Cardiol. 1984 Jan-Feb;79(1):49–58. doi: 10.1007/BF01935806. [DOI] [PubMed] [Google Scholar]
- Schaffer S. W., Safer B., Ford C., Illingworth J., Williamson J. R. Respiratory acidosis and its reversibility in perfused rat heart: regulation of citric acid cycle activity. Am J Physiol. 1978 Jan;234(1):H40–H51. doi: 10.1152/ajpheart.1978.234.1.H40. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Deleeuw G., Barlow C., Chance B., Williamson J. R. Heterogeneity of the hypoxic state in perfused rat heart. Circ Res. 1977 Nov;41(5):606–615. doi: 10.1161/01.res.41.5.606. [DOI] [PubMed] [Google Scholar]
- Tamura M., Hazeki O., Nioka S., Chance B. In vivo study of tissue oxygen metabolism using optical and nuclear magnetic resonance spectroscopies. Annu Rev Physiol. 1989;51:813–834. doi: 10.1146/annurev.ph.51.030189.004121. [DOI] [PubMed] [Google Scholar]
- White R. L., Doeller J. E., Verselis V. K., Wittenberg B. A. Gap junctional conductance between pairs of ventricular myocytes is modulated synergistically by H+ and Ca++. J Gen Physiol. 1990 Jun;95(6):1061–1075. doi: 10.1085/jgp.95.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Doeller J. E., Verselis V. K., Wittenberg B. A. Gap junctional conductance between pairs of ventricular myocytes is modulated synergistically by H+ and Ca++. J Gen Physiol. 1990 Jun;95(6):1061–1075. doi: 10.1085/jgp.95.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg B. A., White R. L., Ginzberg R. D., Spray D. C. Effect of calcium on the dissociation of the mature rat heart into individual and paired myocytes: electrical properties of cell pairs. Circ Res. 1986 Aug;59(2):143–150. doi: 10.1161/01.res.59.2.143. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B. Myoglobin-mediated oxygen delivery to mitochondria of isolated cardiac myocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7503–7507. doi: 10.1073/pnas.84.21.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B. Oxygen pressure gradients in isolated cardiac myocytes. J Biol Chem. 1985 Jun 10;260(11):6548–6554. [PubMed] [Google Scholar]
- Wood E. H. Cardiovascular and pulmonary dynamics by quantitative imaging. Circ Res. 1976 Mar;38(3):131–139. doi: 10.1161/01.res.38.3.131. [DOI] [PubMed] [Google Scholar]


