Abstract
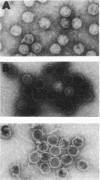
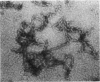
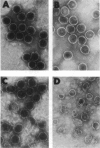
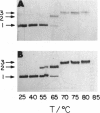
During the packaging of double-stranded DNA by bacterial viruses, the precursor procapsid loses its internal core of scaffolding protein and undergoes a substantial expansion to form the mature virion. Here we show that upon heating, purified P22 procapsids release their scaffolding protein subunits, and the coat protein lattice expands in the absence of any other cellular or viral components. Following these processes by differential scanning calorimetry revealed four different transitions that correlated with structural transitions in the coat protein shells. Exit of scaffolding protein from the procapsid occurred reversibly and just above physiological temperature. Expansion of the procapsid lattice, which was exothermic, occurred after the release of scaffolding protein. Partial denaturation of coat subunits within the intact shell structure was detected prior to the major endothermic event. This major endotherm occurred above 80 degrees C and represents particle breakage and irreversible coat protein denaturation. The results indicate that the coat subunits are designed to form a metastable precursor lattice, which appears to be separated from the mature lattice by a kinetic barrier.
Full text
PDF

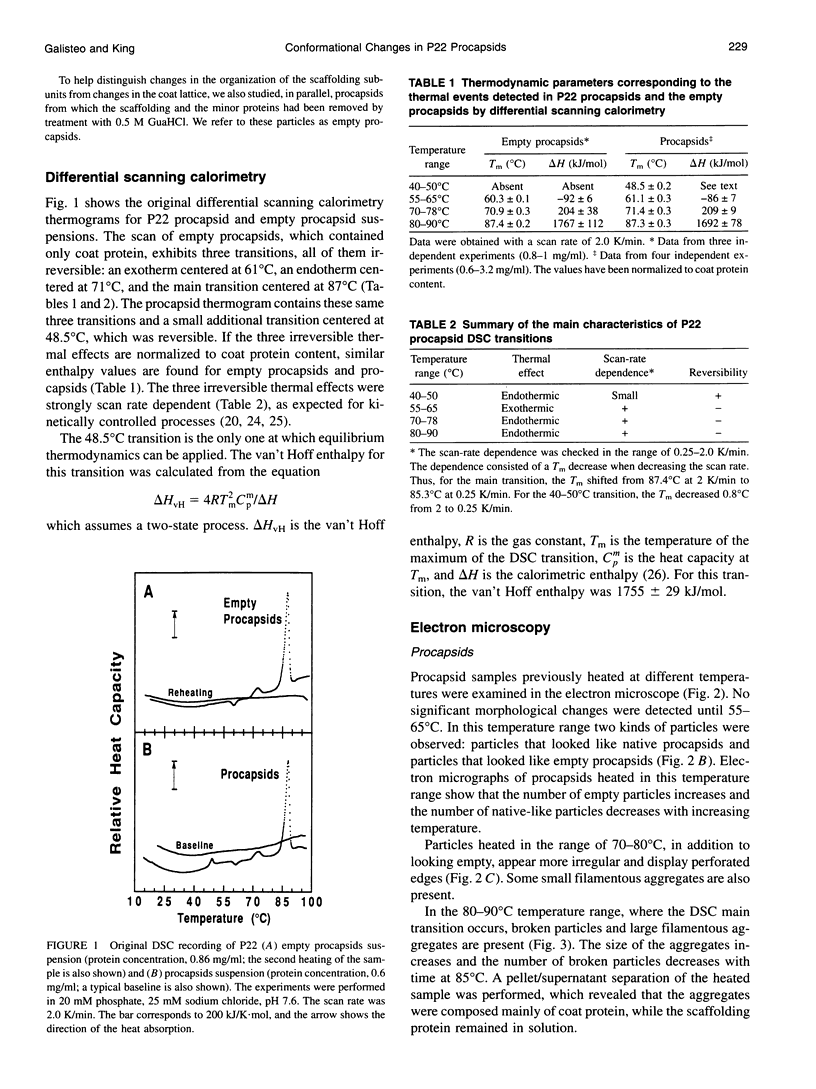
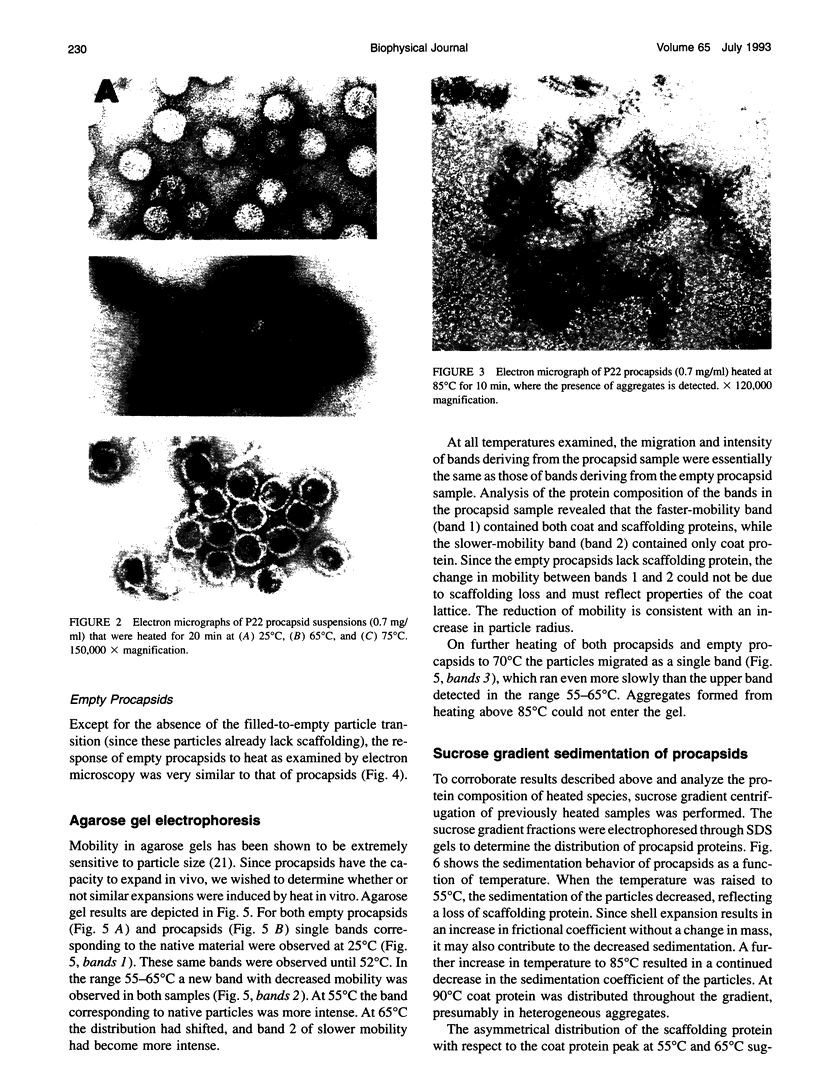
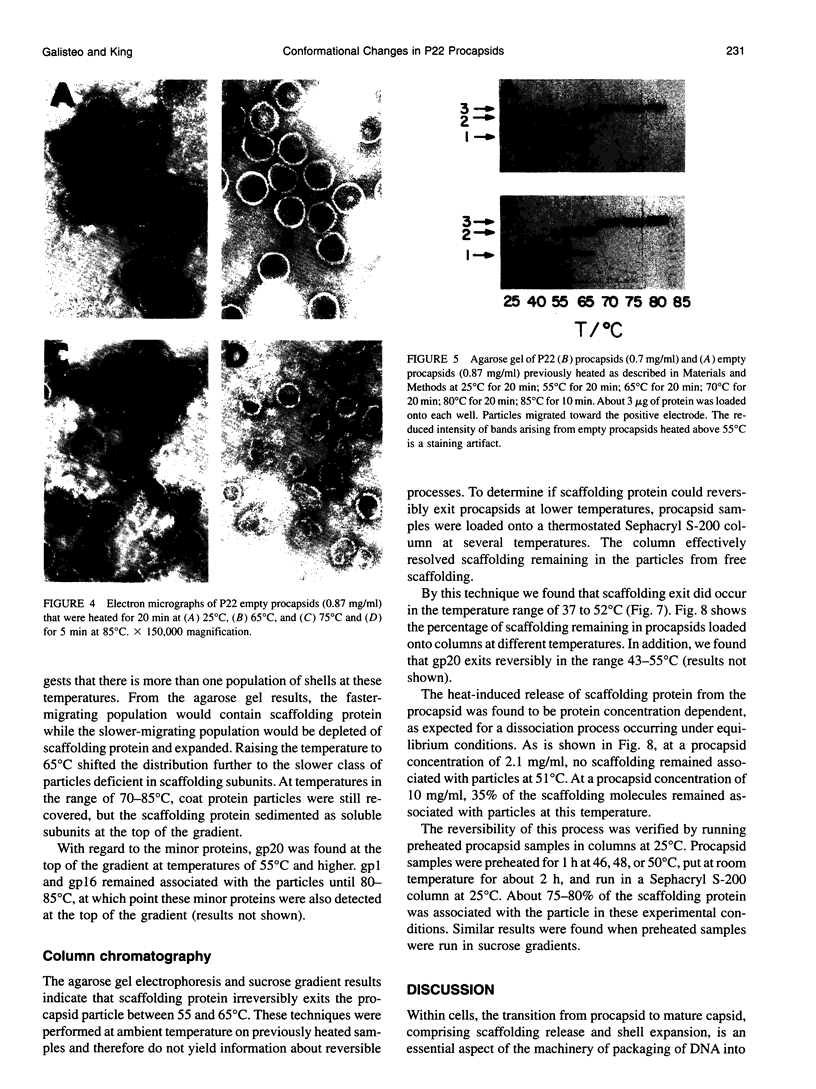
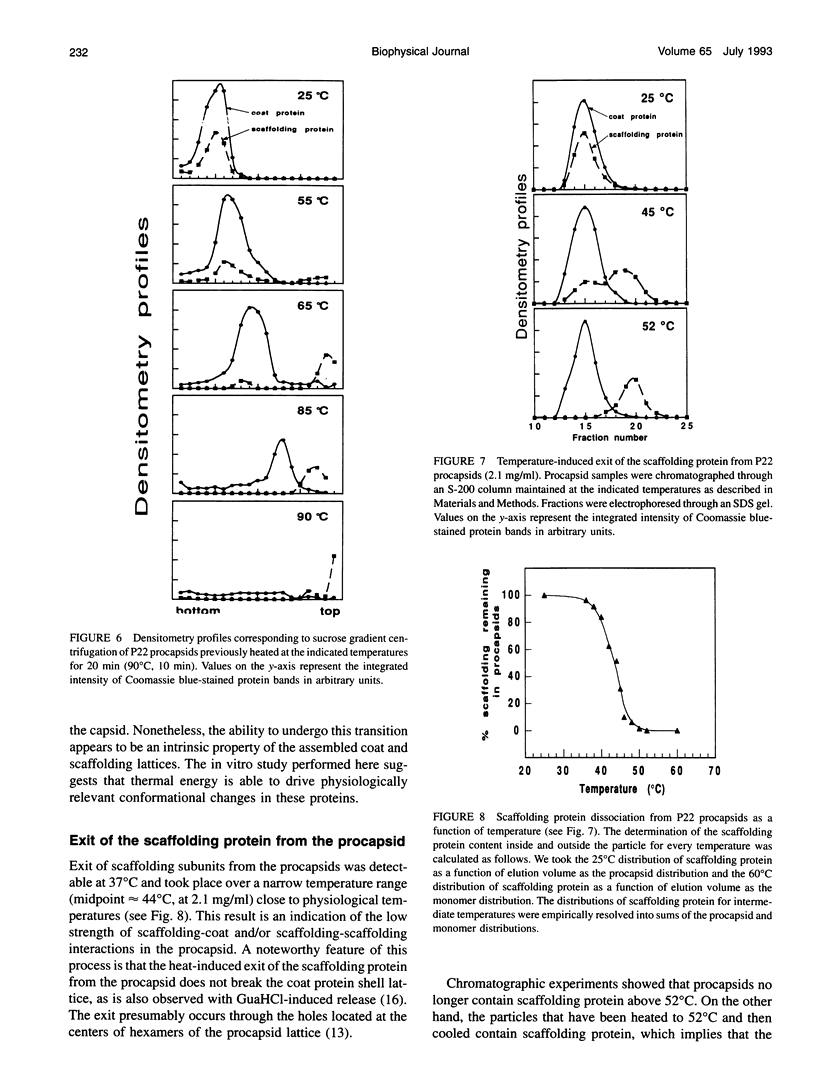



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazinet C., King J. Initiation of P22 procapsid assembly in vivo. J Mol Biol. 1988 Jul 5;202(1):77–86. doi: 10.1016/0022-2836(88)90520-7. [DOI] [PubMed] [Google Scholar]
- Black L. W. DNA packaging in dsDNA bacteriophages. Annu Rev Microbiol. 1989;43:267–292. doi: 10.1146/annurev.mi.43.100189.001411. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Conejero-Lara F., Mateo P. L., Aviles F. X., Sanchez-Ruiz J. M. Effect of Zn2+ on the thermal denaturation of carboxypeptidase B. Biochemistry. 1991 Feb 26;30(8):2067–2072. doi: 10.1021/bi00222a010. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., Casjens S., Harrison S. C. Assembly of the head of bacteriophage P22: x-ray diffraction from heads, proheads and related structures. J Mol Biol. 1976 Jun 25;104(2):387–410. doi: 10.1016/0022-2836(76)90278-3. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., King J. Structure of phage P22 coat protein aggregates formed in the absence of the scaffolding protein. J Mol Biol. 1978 Dec 25;126(4):721–747. doi: 10.1016/0022-2836(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Eppler K., Wyckoff E., Goates J., Parr R., Casjens S. Nucleotide sequence of the bacteriophage P22 genes required for DNA packaging. Virology. 1991 Aug;183(2):519–538. doi: 10.1016/0042-6822(91)90981-g. [DOI] [PubMed] [Google Scholar]
- Fuller M. T., King J. Assembly in vitro of bacteriophage P22 procapsids from purified coat and scaffolding subunits. J Mol Biol. 1982 Apr 15;156(3):633–665. doi: 10.1016/0022-2836(82)90270-4. [DOI] [PubMed] [Google Scholar]
- Fuller M. T., King J. Purification of the coat and scaffolding proteins from procapsids of bacteriophage P22. Virology. 1981 Jul 30;112(2):529–547. doi: 10.1016/0042-6822(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Galisteo M. L., Mateo P. L., Sanchez-Ruiz J. M. Kinetic study on the irreversible thermal denaturation of yeast phosphoglycerate kinase. Biochemistry. 1991 Feb 26;30(8):2061–2066. doi: 10.1021/bi00222a009. [DOI] [PubMed] [Google Scholar]
- Hohn B. DNA sequences necessary for packaging of bacteriophage lambda DNA. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7456–7460. doi: 10.1073/pnas.80.24.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Wurtz M., Klein B., Lustig A., Hohn T. Phage lambda DNA packaging, in vitro. J Supramol Struct. 1974;2(2-4):302–317. doi: 10.1002/jss.400020220. [DOI] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Amos L. A., Klug A. Correlation between structural transformation and cleavage of the major head protein of T4 bacteriophage. Cell. 1976 Feb;7(2):191–203. doi: 10.1016/0092-8674(76)90018-0. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Prevelige P. E., Marietta E., Chen R. O., Thomas D., King J., Chiu W. Three-dimensional transformation of capsids associated with genome packaging in a bacterial virus. J Mol Biol. 1993 May 5;231(1):65–74. doi: 10.1006/jmbi.1993.1257. [DOI] [PubMed] [Google Scholar]
- Prevelige P. E., Jr, Thomas D., King J. Nucleation and growth phases in the polymerization of coat and scaffolding subunits into icosahedral procapsid shells. Biophys J. 1993 Mar;64(3):824–835. doi: 10.1016/S0006-3495(93)81443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevelige P. E., Jr, Thomas D., King J. Scaffolding protein regulates the polymerization of P22 coat subunits into icosahedral shells in vitro. J Mol Biol. 1988 Aug 20;202(4):743–757. doi: 10.1016/0022-2836(88)90555-4. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Potekhin S. A. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods Enzymol. 1986;131:4–51. doi: 10.1016/0076-6879(86)31033-4. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Rao V. B., Black L. W. DNA packaging of bacteriophage T4 proheads in vitro. Evidence that prohead expansion is not coupled to DNA packaging. J Mol Biol. 1985 Oct 5;185(3):565–578. doi: 10.1016/0022-2836(85)90072-5. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Black L. W., Bisher M. E., Steven A. C. Assembly-dependent conformational changes in a viral capsid protein. Calorimetric comparison of successive conformational states of the gp23 surface lattice of bacteriophage T4. J Mol Biol. 1985 Jun 5;183(3):353–364. doi: 10.1016/0022-2836(85)90006-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ruiz J. M. Theoretical analysis of Lumry-Eyring models in differential scanning calorimetry. Biophys J. 1992 Apr;61(4):921–935. doi: 10.1016/S0006-3495(92)81899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwer P. A metrizamide-impermeable capsid in the DNA packaging pathway of bacteriophage T7. J Mol Biol. 1980 Mar 25;138(1):65–91. doi: 10.1016/s0022-2836(80)80005-2. [DOI] [PubMed] [Google Scholar]
- Serwer P., Hayes S. J., Griess G. A. Determination of a particle's radius by two-dimensional agarose gel electrophoresis. Anal Biochem. 1986 Feb 1;152(2):339–345. doi: 10.1016/0003-2697(86)90419-7. [DOI] [PubMed] [Google Scholar]
- Serwer P., Pichler M. E. Electrophoresis of bacteriophage T7 and T7 capsids in agarose gels. J Virol. 1978 Dec;28(3):917–928. doi: 10.1128/jvi.28.3.917-928.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H., Fujisawa H., Minagawa T. Characterization of the bacteriophage T3 DNA packaging reaction in vitro in a defined system. J Mol Biol. 1987 Aug 20;196(4):845–851. doi: 10.1016/0022-2836(87)90409-8. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ruiz J. M., López-Lacomba J. L., Cortijo M., Mateo P. L. Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry. 1988 Mar 8;27(5):1648–1652. doi: 10.1021/bi00405a039. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Casey J. L., Sturtevant J. M. Thermodynamics of the binding of D-glucose to yeast hexokinase. Biochemistry. 1981 Aug 4;20(16):4693–4697. doi: 10.1021/bi00519a026. [DOI] [PubMed] [Google Scholar]