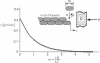
Abstract
We present here a model for how chemical reactions generate protrusive forces by rectifying Brownian motion. This sort of energy transduction drives a number of intracellular processes, including filopodial protrusion, propulsion of the bacterium Listeria, and protein translocation.
Full text
PDF
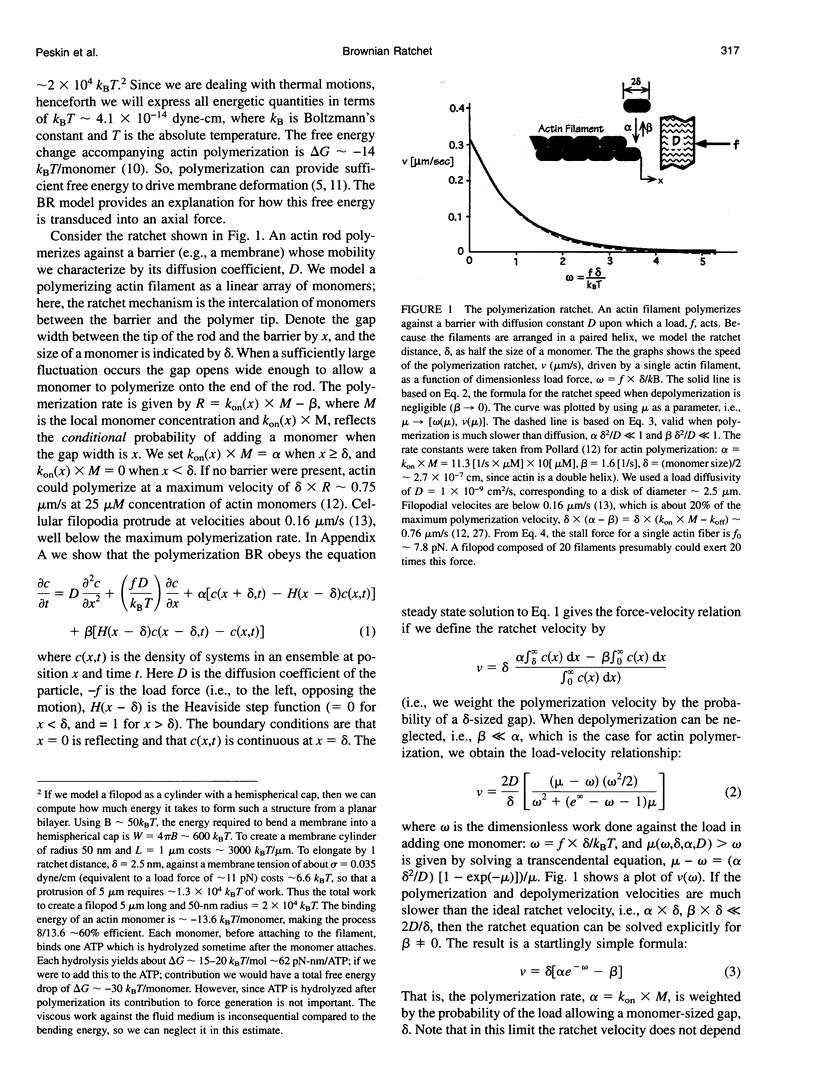





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argiro V., Bunge M. B., Johnson M. I. A quantitative study of growth cone filopodial extension. J Neurosci Res. 1985;13(1-2):149–162. doi: 10.1002/jnr.490130111. [DOI] [PubMed] [Google Scholar]
- Bo L., Waugh R. E. Determination of bilayer membrane bending stiffness by tether formation from giant, thin-walled vesicles. Biophys J. 1989 Mar;55(3):509–517. doi: 10.1016/S0006-3495(89)82844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., Money N. P., Harold F. M., Bamburg J. R. Responses of growth cones to changes in osmolality of the surrounding medium. J Cell Sci. 1991 Apr;98(Pt 4):507–515. doi: 10.1242/jcs.98.4.507. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. The role of actin polymerization in cell motility. Annu Rev Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- Córdova N. J., Ermentrout B., Oster G. F. Dynamics of single-motor molecules: the thermal ratchet model. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):339–343. doi: 10.1073/pnas.89.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri G. A., Sanger J. M., Portnoy D. A., Southwick F. S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P., Lin C. H., Thompson C. Novel form of growth cone motility involving site-directed actin filament assembly. Nature. 1992 Jun 11;357(6378):515–518. doi: 10.1038/357515a0. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Yang Y. Z., Korn E. D. Polymerization of Acanthamoeba actin. Kinetics, thermodynamics, and co-polymerization with muscle actin. J Biol Chem. 1976 Dec 10;251(23):7474–7479. [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hill T. L., Kirschner M. W. Bioenergetics and kinetics of microtubule and actin filament assembly-disassembly. Int Rev Cytol. 1982;78:1–125. [PubMed] [Google Scholar]
- Hill T. L., Kirschner M. W. Regulation of microtubule and actin filament assembly--disassembly by associated small and large molecules. Int Rev Cytol. 1983;84:185–234. doi: 10.1016/s0074-7696(08)61018-9. [DOI] [PubMed] [Google Scholar]
- Inoué S., Tilney L. G. Acrosomal reaction of thyone sperm. I. Changes in the sperm head visualized by high resolution video microscopy. J Cell Biol. 1982 Jun;93(3):812–819. doi: 10.1083/jcb.93.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Berg H. C. Isotope and thermal effects in chemiosmotic coupling to the flagellar motor of Streptococcus. Cell. 1983 Mar;32(3):913–919. doi: 10.1016/0092-8674(83)90076-4. [DOI] [PubMed] [Google Scholar]
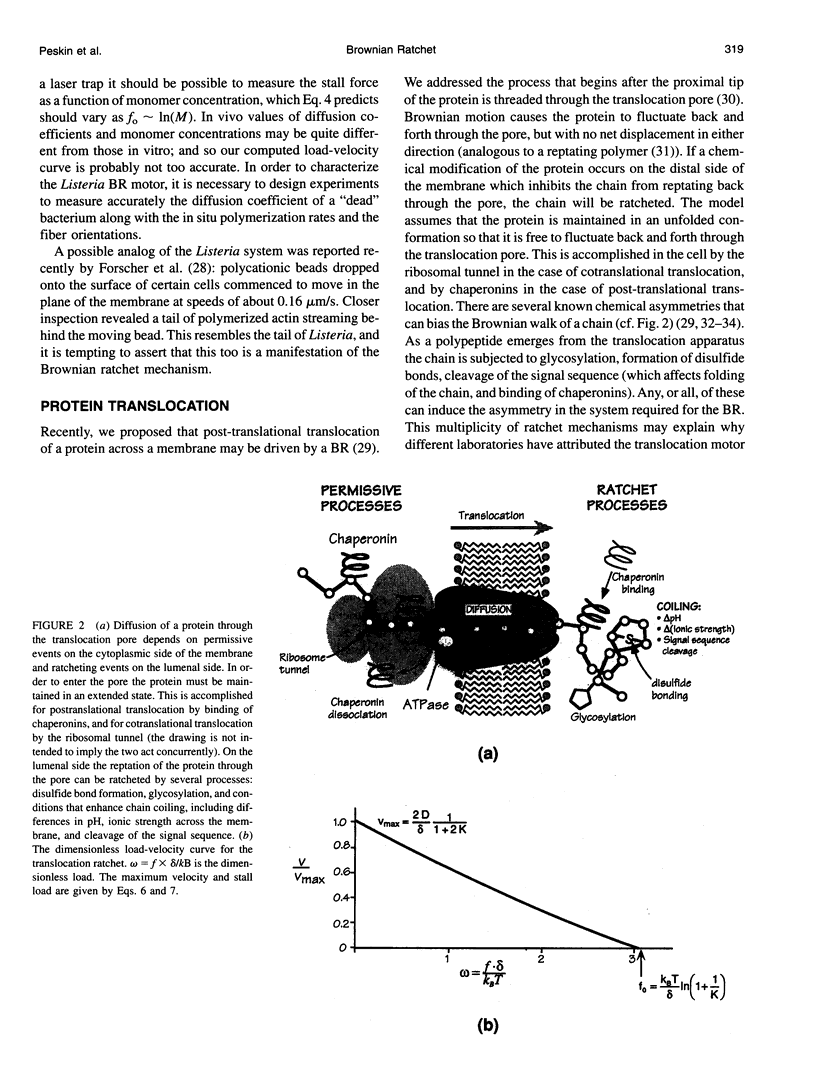
- Koshland D. E., Mitchison T. J., Kirschner M. W. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988 Feb 11;331(6156):499–504. doi: 10.1038/331499a0. [DOI] [PubMed] [Google Scholar]
- Leibler S., Huse D. A. A physical model for motor proteins. C R Acad Sci III. 1991;313(1):27–35. [PubMed] [Google Scholar]
- Liu S. C., Derick L. H., Zhai S., Palek J. Uncoupling of the spectrin-based skeleton from the lipid bilayer in sickled red cells. Science. 1991 Apr 26;252(5005):574–576. doi: 10.1126/science.2020854. [DOI] [PubMed] [Google Scholar]
- Meister M., Caplan S. R., Berg H. C. Dynamics of a tightly coupled mechanism for flagellar rotation. Bacterial motility, chemiosmotic coupling, protonmotive force. Biophys J. 1989 May;55(5):905–914. doi: 10.1016/S0006-3495(89)82889-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui T., Ohshima H. A self-induced translation model of myosin head motion in contracting muscle. I. Force-velocity relation and energy liberation. J Muscle Res Cell Motil. 1988 Jun;9(3):248–260. doi: 10.1007/BF01773895. [DOI] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J. Bidirectional movement of a nascent polypeptide across microsomal membranes reveals requirements for vectorial translocation of proteins. Cell. 1992 Oct 2;71(1):87–96. doi: 10.1016/0092-8674(92)90268-h. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Actin. Curr Opin Cell Biol. 1990 Feb;2(1):33–40. doi: 10.1016/s0955-0674(05)80028-6. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J Cell Biol. 1986 Dec;103(6 Pt 2):2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. M., Sanger J. W., Southwick F. S. Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect Immun. 1992 Sep;60(9):3609–3619. doi: 10.1128/iai.60.9.3609-3619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Blobel G. A protein-conducting channel in the endoplasmic reticulum. Cell. 1991 May 3;65(3):371–380. doi: 10.1016/0092-8674(91)90455-8. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Peskin C. S., Oster G. F. What drives the translocation of proteins? Proc Natl Acad Sci U S A. 1992 May 1;89(9):3770–3774. doi: 10.1073/pnas.89.9.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J., Tilney L. G., Portnoy D. A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992 May 21;357(6375):257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Inoué S. Acrosomal reaction of Thyone sperm. II. The kinetics and possible mechanism of acrosomal process elongation. J Cell Biol. 1982 Jun;93(3):820–827. doi: 10.1083/jcb.93.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Inoué S. Acrosomal reaction of the Thyone sperm. III. The relationship between actin assembly and water influx during the extension of the acrosomal process. J Cell Biol. 1985 Apr;100(4):1273–1283. doi: 10.1083/jcb.100.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Oosawa F. Protein motors and Maxwell's demons: does mechanochemical transduction involve a thermal ratchet? Adv Biophys. 1990;26:97–134. doi: 10.1016/0065-227x(90)90009-i. [DOI] [PubMed] [Google Scholar]