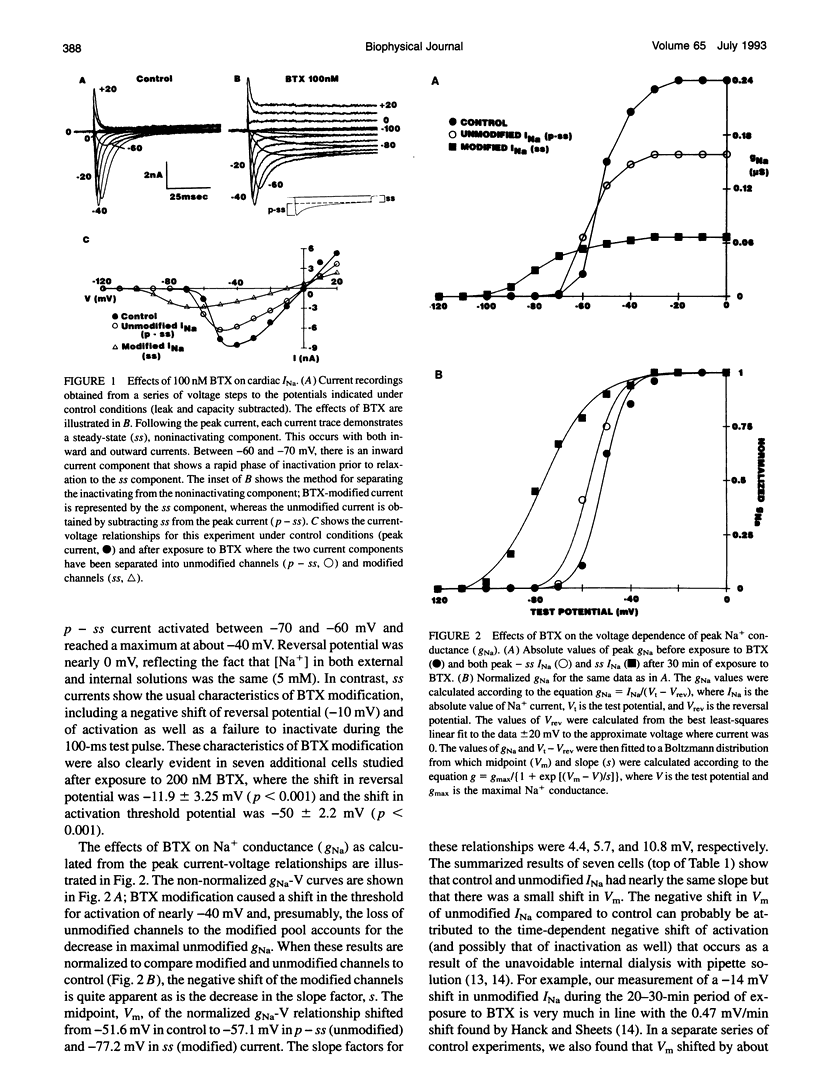
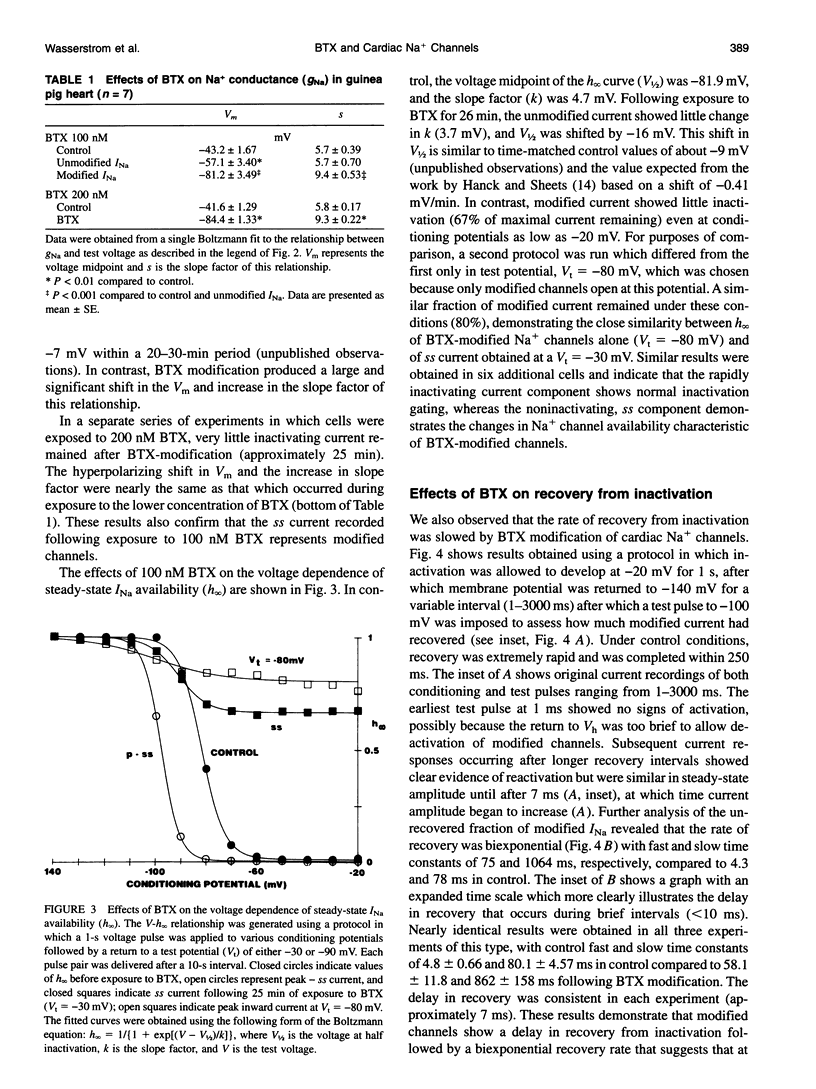
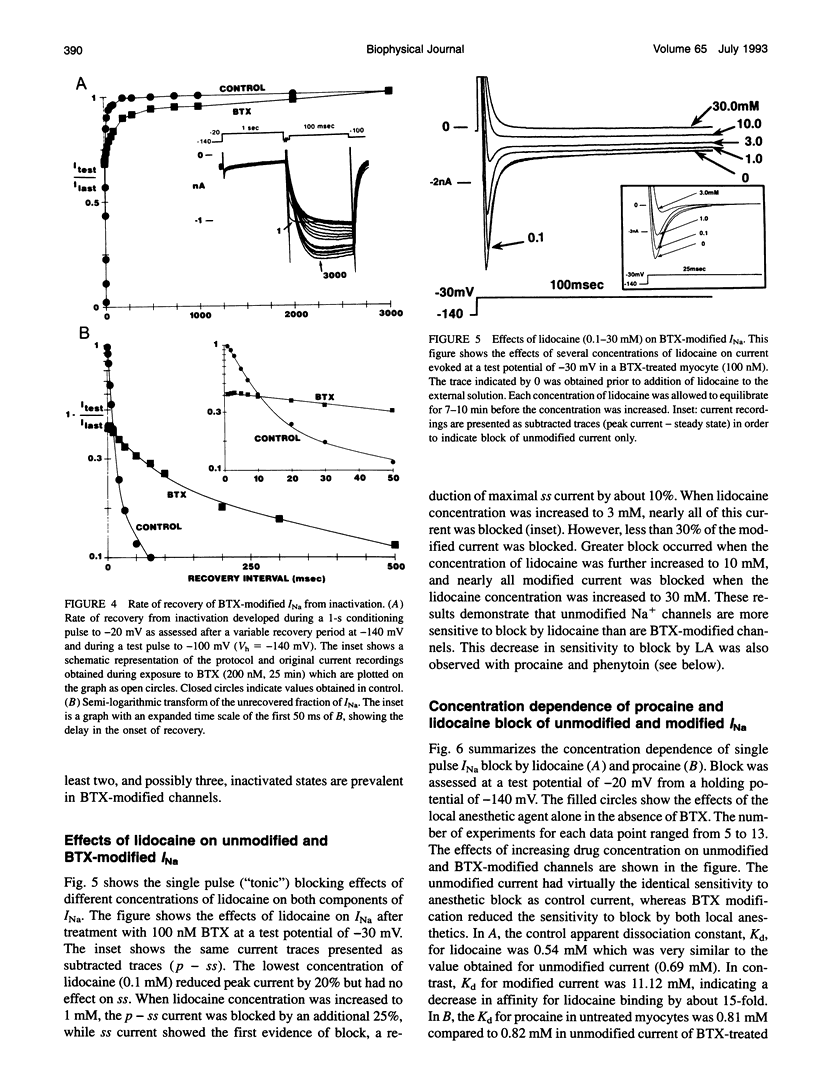
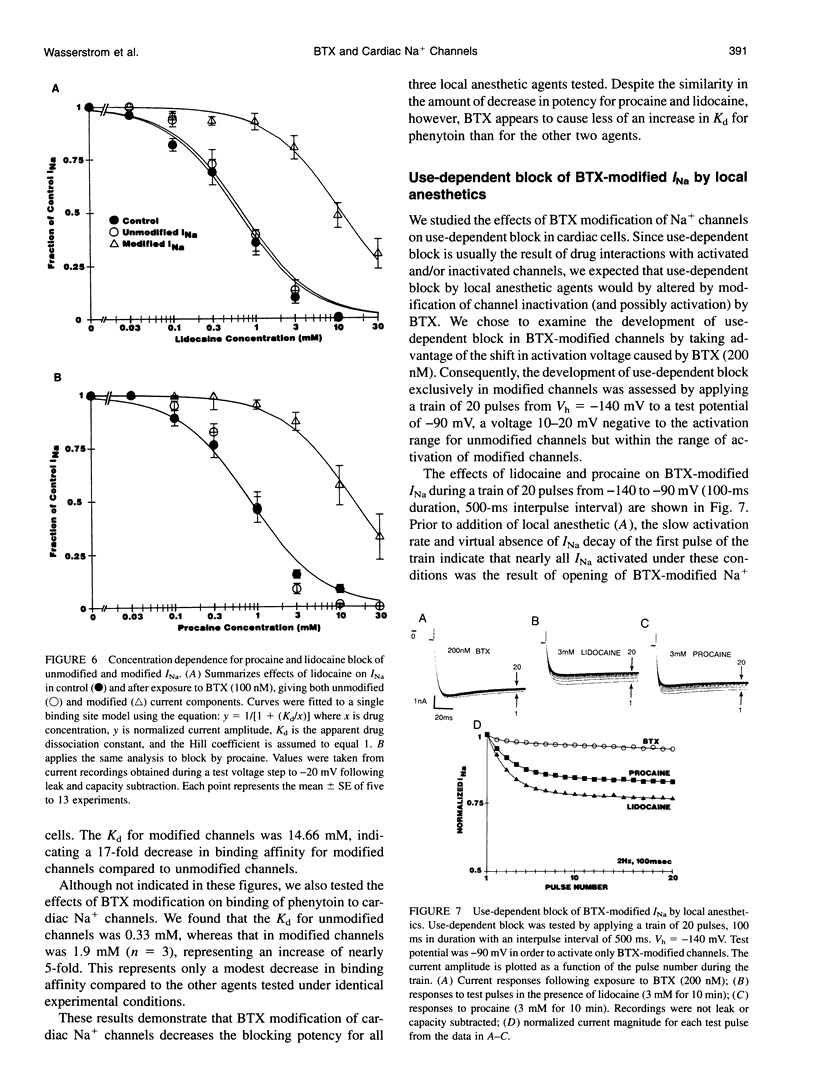
Abstract
The purpose of the present study was to examine the characteristics of Na+ channel modification by batrachotoxin (BTX) in cardiac cells, including changes in channel gating and kinetics as well as susceptibility to block by local anesthetic agents. We used the whole cell configuration of the patch clamp technique to measure Na+ current in guinea pig myocytes. Extracellular Na+ concentration and temperature were lowered (5-10 mM, 17 degrees C) in order to maintain good voltage control. Our results demonstrated that 1) BTX modifies cardiac INa, causing a substantial steady-state (noninactivating) component of INa, 2) modification of cardiac Na+ channels by BTX shifts activation to more negative potentials and reduces both maximal gNa and selectivity for Na+; 3) binding of BTX to its receptor in the cardiac Na+ channel reduces the affinity of local anesthetics for their binding site; and 4) BTX-modified channels show use-dependent block by local anesthetics. The reduced blocking potency of local anesthetics for BTX-modified Na+ channels probably results from an allosteric interaction between BTX and local anesthetics for their respective binding sites in the Na+ channel. Our observations that use-dependent block by local anesthetics persists in BTX-modified Na+ channels suggest that this form of extra block can occur in the virtual absence of the inactivated state. Thus, the development of use-dependent block appears to rely primarily on local anesthetic binding to activated Na+ channels under these conditions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979 Jul;27(1):39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Inhibition of voltage-sensitive sodium channels in neuroblastoma cells by antiarrhythmic drugs. Mol Pharmacol. 1981 Sep;20(2):356–362. [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W., Follmer C. H., Ten Eick R. E., Hondeghem L. M., Yeh J. Z. Evidence for two components of sodium channel block by lidocaine in isolated cardiac myocytes. Circ Res. 1988 Nov;63(5):869–878. doi: 10.1161/01.res.63.5.869. [DOI] [PubMed] [Google Scholar]
- Creveling C. R., McNeal E. T., Daly J. W., Brown G. B. Batrachotoxin-induced depolarization and [3H]batrachotoxinin-a 20 alpha-benzoate binding in a vesicular preparation from guinea pig cerebral cortex. Mol Pharmacol. 1983 Mar;23(2):350–358. [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanck D. A., Sheets M. F. Time-dependent changes in kinetics of Na+ current in single canine cardiac Purkinje cells. Am J Physiol. 1992 Apr;262(4 Pt 2):H1197–H1207. doi: 10.1152/ajpheart.1992.262.4.H1197. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan P. M., Albuquerque E. X. The pharmacology of batrachotoxin. 3. Effect on the heart Purkinje fibers. J Pharmacol Exp Ther. 1971 Mar;176(3):529–537. [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Honerjäger P., Reiter M. The cardiotoxic effect of batrachotoxin. Naunyn Schmiedebergs Arch Pharmacol. 1977 Oct;299(3):239–252. doi: 10.1007/BF00500316. [DOI] [PubMed] [Google Scholar]
- Huang L. M., Ehrenstein G. Local anesthetics QX 572 and benzocaine act at separate sites on the batrachotoxin-activated sodium channel. J Gen Physiol. 1981 Feb;77(2):137–153. doi: 10.1085/jgp.77.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y., Moran N., Ehrenstein G. Batrachotoxin modifies the gating kinetics of sodium channels in internally perfused neuroblastoma cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2082–2085. doi: 10.1073/pnas.79.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y., Yatani A., Brown A. M. The properties of batrachotoxin-modified cardiac Na channels, including state-dependent block by tetrodotoxin. J Gen Physiol. 1987 Sep;90(3):341–360. doi: 10.1085/jgp.90.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorov B. I. Batrachotoxin as a tool to study voltage-sensitive sodium channels of excitable membranes. Prog Biophys Mol Biol. 1985;45(2):57–148. doi: 10.1016/0079-6107(85)90005-7. [DOI] [PubMed] [Google Scholar]
- Khodorov B. I., Peganov E. M., Revenko S. V., Shishkova L. D. Sodium currents in voltage clamped nerve fiber of frog under the combined action of batrachotoxin and procaine. Brain Res. 1975 Feb 14;84(3):541–546. doi: 10.1016/0006-8993(75)90771-4. [DOI] [PubMed] [Google Scholar]
- Khodorov B. I., Revenko S. V. Further analysis of the mechanisms of action of batrachotoxin on the membrane of myelinated nerve. Neuroscience. 1979;4(9):1315–1330. doi: 10.1016/0306-4522(79)90159-3. [DOI] [PubMed] [Google Scholar]
- Khodorov B. I., Zaborovskaya L. D. Blockade of sodium and potassium channels in the node of Ranvier by ajmaline and N-propyl ajmaline. Gen Physiol Biophys. 1983 Aug;2(4):233–268. [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Quandt F. N., Narahashi T. Modification of single Na+ channels by batrachotoxin. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6732–6736. doi: 10.1073/pnas.79.21.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Chapula J., Tsuda Y., Josephson I. R. Voltage- and use-dependent effects of lidocaine on sodium current in rat single ventricular cells. Circ Res. 1983 May;52(5):557–565. doi: 10.1161/01.res.52.5.557. [DOI] [PubMed] [Google Scholar]
- Starmer C. F., Grant A. O., Strauss H. C. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J. 1984 Jul;46(1):15–27. doi: 10.1016/S0006-3495(84)83994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguy J., Yeh J. Z. BTX modification of Na channels in squid axons. I. State dependence of BTX action. J Gen Physiol. 1991 Mar;97(3):499–519. doi: 10.1085/jgp.97.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguy J., Yeh J. Z. Batrachotoxin uncouples gating charge immobilization from fast Na inactivation in squid giant axons. Biophys J. 1988 Oct;54(4):719–730. doi: 10.1016/S0006-3495(88)83007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguy J., Yeh J. Z. QX-314 restores gating charge immobilization abolished by chloramine-T treatment in squid giant axons. Biophys J. 1989 Aug;56(2):421–427. doi: 10.1016/S0006-3495(89)82688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K., Brodwick M. S., Eaton D. C., Strichartz G. R. Inhibition of sodium currents by local anesthetics in chloramine-T-treated squid axons. The role of channel activation. J Gen Physiol. 1987 Apr;89(4):645–667. doi: 10.1085/jgp.89.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K., Wang S. Y. Inactivation of batrachotoxin-modified Na+ channels in GH3 cells. Characterization and pharmacological modification. J Gen Physiol. 1992 Jan;99(1):1–20. doi: 10.1085/jgp.99.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willow M., Catterall W. A. Inhibition of binding of [3H]batrachotoxinin A 20-alpha-benzoate to sodium channels by the anticonvulsant drugs diphenylhydantoin and carbamazepine. Mol Pharmacol. 1982 Nov;22(3):627–635. [PubMed] [Google Scholar]
- Yeh J. Z. A pharmacological approach to the structure of the Na channel in squid axon. Prog Clin Biol Res. 1982;79:17–49. [PubMed] [Google Scholar]


