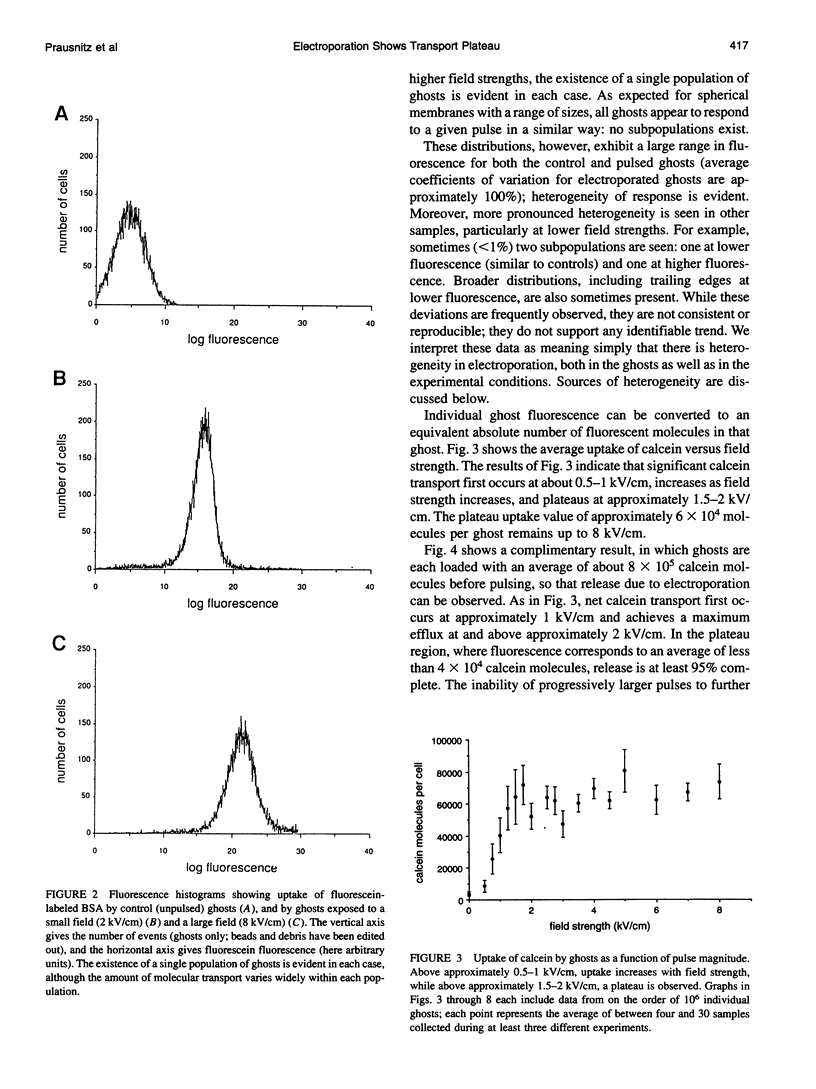
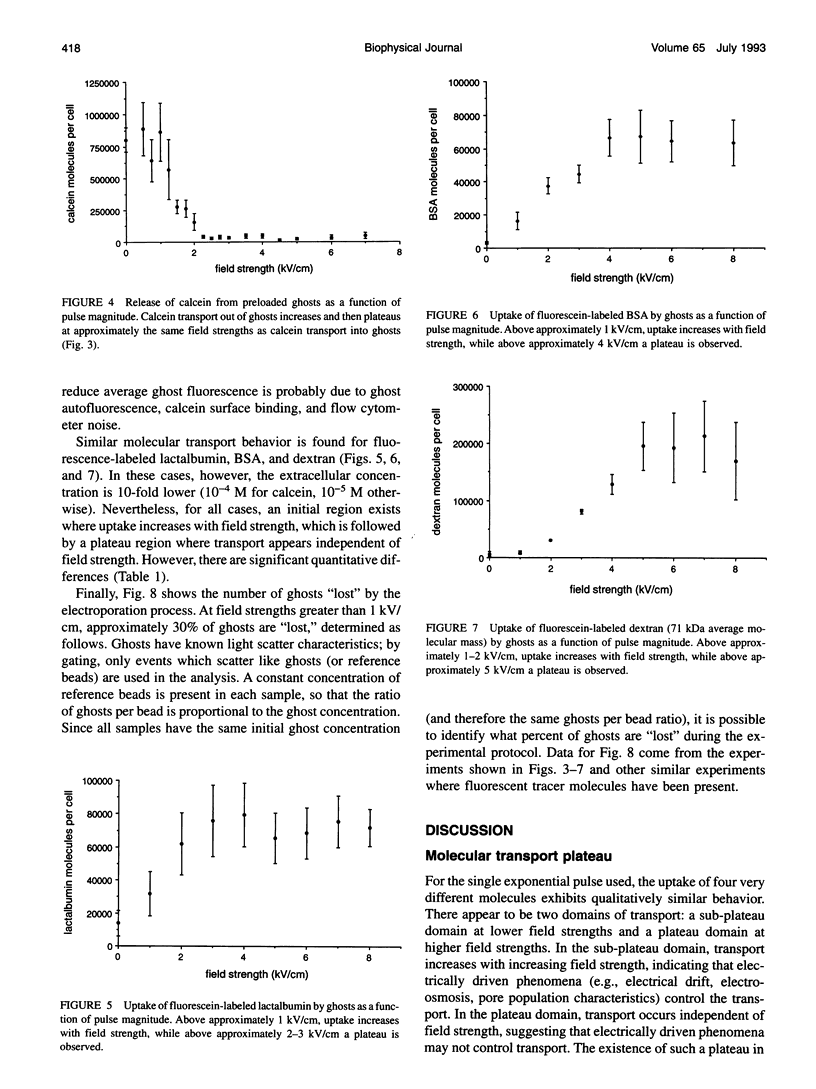
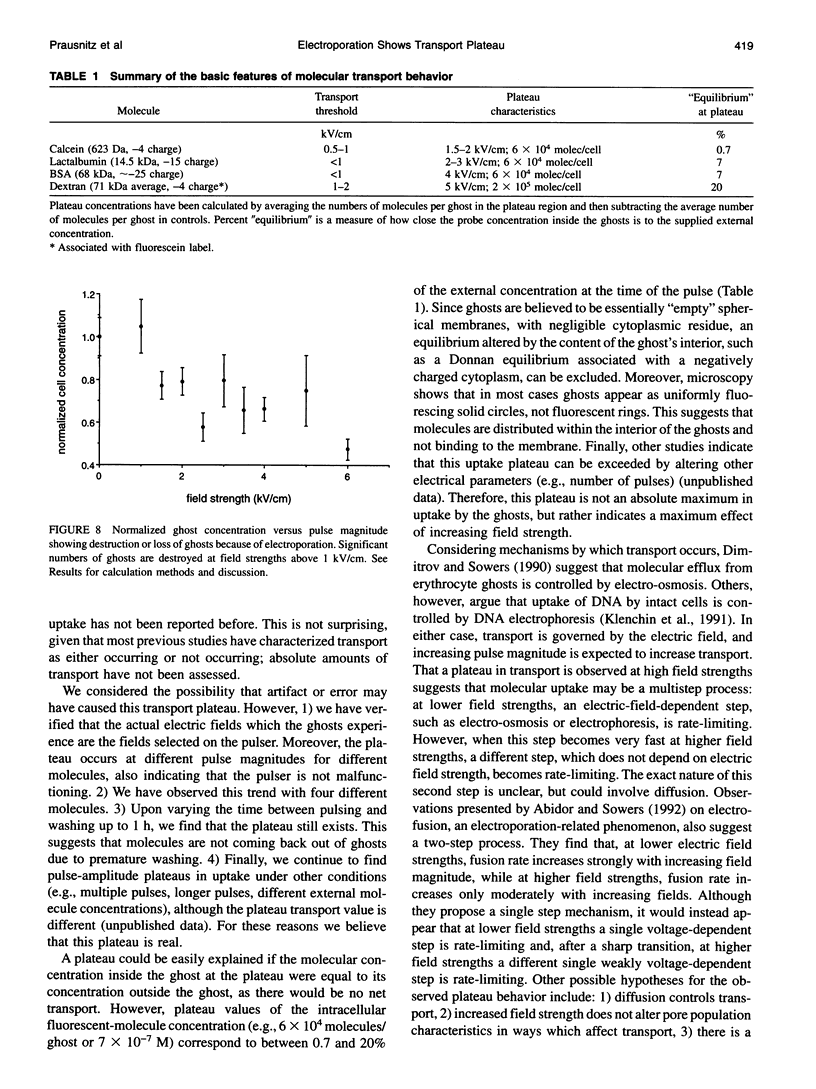
Abstract
Electroporation is believed to involve a temporary structural rearrangement of lipid bilayer membranes, which results in ion and molecular transport across the membrane. The results of a quantitative study of molecular transport due to electroporation caused by a single exponential pulse are presented; transport of four molecules of different physical characteristics across erythrocyte ghost membranes is examined as a function of applied field strength. Flow cytometry is used to quantitatively measure the number of molecules transported for 10(4) to 10(5) individual ghosts for each condition. This study has four major findings: 1) Net transport first increases with field strength, but reaches a plateau at higher field strengths. Significant transport is found at or below 1 kV/cm, and transport plateaus begin at field strengths between 2 and 5 kV/cm depending on the molecule transported. 2) A single population of ghosts generally exists, but exhibits a wide distribution in the amount of molecular transport. 3) Under the conditions used, the direction of transport across the ghost membrane does not appear to affect molecular transport significantly. 4) Large numbers of ghosts may be destroyed by the electroporation procedure.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abidor I. G., Sowers A. E. Kinetics and mechanism of cell membrane electrofusion. Biophys J. 1992 Jun;61(6):1557–1569. doi: 10.1016/S0006-3495(92)81960-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer D., Brandner G., Bodemer W. Dielectric breakdown of the red blood cell membrane and uptake of SV 40 DNA and mammalian cell RNA. Naturwissenschaften. 1976 Aug;63(8):391–391. doi: 10.1007/BF00607946. [DOI] [PubMed] [Google Scholar]
- Bartoletti D. C., Harrison G. I., Weaver J. C. The number of molecules taken up by electroporated cells: quantitative determination. FEBS Lett. 1989 Oct 9;256(1-2):4–10. doi: 10.1016/0014-5793(89)81707-7. [DOI] [PubMed] [Google Scholar]
- Benz R., Beckers F., Zimmermann U. Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. J Membr Biol. 1979 Jul 16;48(2):181–204. doi: 10.1007/BF01872858. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R., Wylie D. E., Schuster S. M. Transfer of monoclonal antibodies into mammalian cells by electroporation. J Biol Chem. 1989 Sep 15;264(26):15494–15500. [PubMed] [Google Scholar]
- Chang D. C., Reese T. S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990 Jul;58(1):1–12. doi: 10.1016/S0006-3495(90)82348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Sowers A. E. Membrane electroporation--fast molecular exchange by electroosmosis. Biochim Biophys Acta. 1990 Mar;1022(3):381–392. doi: 10.1016/0005-2736(90)90289-z. [DOI] [PubMed] [Google Scholar]
- Hibino M., Shigemori M., Itoh H., Nagayama K., Kinosita K., Jr Membrane conductance of an electroporated cell analyzed by submicrosecond imaging of transmembrane potential. Biophys J. 1991 Jan;59(1):209–220. doi: 10.1016/S0006-3495(91)82212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977 Aug 4;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- Klenchin V. A., Sukharev S. I., Serov S. M., Chernomordik L. V., Chizmadzhev YuA Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys J. 1991 Oct;60(4):804–811. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H., Pankov R., Gauthier J., Hancock R. Electroporation-mediated uptake of proteins into mammalian cells. Biochem Cell Biol. 1990 Apr;68(4):729–734. doi: 10.1139/o90-105. [DOI] [PubMed] [Google Scholar]
- Lange Y., Gough A., Steck T. L. Role of the bilayer in the shape of the isolated erythrocyte membrane. J Membr Biol. 1982;69(2):113–123. doi: 10.1007/BF01872271. [DOI] [PubMed] [Google Scholar]
- Liang H., Purucker W. J., Stenger D. A., Kubiniec R. T., Hui S. W. Uptake of fluorescence-labeled dextrans by 10T 1/2 fibroblasts following permeation by rectangular and exponential-decay electric field pulses. Biotechniques. 1988 Jun;6(6):550-2, 554, 556-8. [PubMed] [Google Scholar]
- Lieber M. R., Steck T. L. A description of the holes in human erythrocyte membrane ghosts. J Biol Chem. 1982 Oct 10;257(19):11651–11659. [PubMed] [Google Scholar]
- Lieber M. R., Steck T. L. Dynamics of the holes in human erythrocyte membrane ghosts. J Biol Chem. 1982 Oct 10;257(19):11660–11666. [PubMed] [Google Scholar]
- Mir L. M., Banoun H., Paoletti C. Introduction of definite amounts of nonpermeant molecules into living cells after electropermeabilization: direct access to the cytosol. Exp Cell Res. 1988 Mar;175(1):15–25. doi: 10.1016/0014-4827(88)90251-0. [DOI] [PubMed] [Google Scholar]
- Poddevin B., Orlowski S., Belehradek J., Jr, Mir L. M. Very high cytotoxicity of bleomycin introduced into the cytosol of cells in culture. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S67–S75. doi: 10.1016/0006-2952(91)90394-k. [DOI] [PubMed] [Google Scholar]
- Rosemberg Y., Korenstein R. Electroporation of the photosynthetic membrane: A study by intrinsic and external optical probes. Biophys J. 1990 Oct;58(4):823–832. doi: 10.1016/S0006-3495(90)82428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A. J., Hamilton W. A. Effects of high electric fields on micro-organisms. 3. Lysis of erythrocytes and protoplasts. Biochim Biophys Acta. 1968 Aug;163(1):37–43. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- Sowers A. E., Lieber M. R. Electropore diameters, lifetimes, numbers, and locations in individual erythrocyte ghosts. FEBS Lett. 1986 Sep 15;205(2):179–184. doi: 10.1016/0014-5793(86)80893-6. [DOI] [PubMed] [Google Scholar]
- Tekle E., Astumian R. D., Chock P. B. Electroporation by using bipolar oscillating electric field: an improved method for DNA transfection of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4230–4234. doi: 10.1073/pnas.88.10.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J. C., Harrison G. I., Bliss J. G., Mourant J. R., Powell K. T. Electroporation: high frequency of occurrence of a transient high-permeability state in erythrocytes and intact yeast. FEBS Lett. 1988 Feb 29;229(1):30–34. doi: 10.1016/0014-5793(88)80791-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Riemann F., Pilwat G. Enzyme loading of electrically homogeneous human red blood cell ghosts prepared by dielelctric breakdown. Biochim Biophys Acta. 1976 Jun 17;436(2):460–474. doi: 10.1016/0005-2736(76)90208-x. [DOI] [PubMed] [Google Scholar]


