Abstract
p53 mediates apoptosis of cells after DNA damage including tumor cells after radiation or chemotherapy. Survival of isolated cancer cells after therapy leads to recurrence of therapy-resistant tumors. We now show that for some melanoma, sarcoma, or fibroblastic cell types that survive without integrin-mediated adhesion, apoptosis in response to DNA damage requires cell adhesion. This effect correlates with rapid changes in levels of p14/p19 Arf and its downstream component, p53. Killing of nonadherent cells is increased by treatment with antiintegrin antibodies or by increasing levels of p53 or Arf. Consistent with low p53 levels, suspended cells show chromosomal instability after irradiation. Thus, loss of normal adhesion in susceptible tumor cells during genotoxic stress may play a role in therapy resistance and promote survival of cells with aberrant DNA.
Keywords: p14 Arf‖apoptosis‖cancer‖extracellular matrix
Apoptosis is a key component in the development and maintenance of tissues within mammalian organisms, providing a mechanism to eliminate damaged or unnecessary cells (1–3). Conventional cancer therapies take advantage of this mechanism by employing ionizing radiation or chemicals to damage DNA and induce selective apoptosis of rapidly growing cells. However, killing of tumor cells commonly is incomplete, allowing tumor recurrence and progression toward more aggressive phenotypes. Understanding the molecular mechanisms by which DNA damage induces cells to undergo apoptosis therefore is an important goal.
Apoptosis in response to DNA damage involves a complex pathway in which p53 and p53 family members play critical regulatory roles. P53 is a tumor suppressor protein that serves as a molecular switch between different cell fates after DNA damage and other stresses, leading to either apoptosis or cell-cycle arrest (1–3). The importance of p53 is underscored by the fact that most human cancers are found to have lost this pathway and that its loss is a critical event in the transition to therapy resistance (1).
p53 normally is maintained at low levels largely because of Mdm2-mediated degradation (4, 5). As few as one double-strand break (6) can trigger events that decrease the interaction of p53 with Mdm2 to increase p53 levels. Phosphorylation of p53 at serines 15 and 20 by ATM and CHK2, respectively, block binding to Mdm2 to stabilize p53 (7). Additionally, increased p19Arf inhibits the action of Mdm2 by sequestering it within nucleoli to increase p53 levels (4, 8). Increased p53 results in transactivation of a number of targets including genes that control cell growth, apoptosis, and DNA repair (9–11). After DNA damage, p53 mediates a delay in the G1 phase of the cell cycle to permit DNA repair to occur (12, 13). Alternatively, if DNA damage is severe, p53 promotes apoptosis (1).
Integrins are receptors that mediate attachment and spreading on extracellular matrix (ECM) proteins and transduce signals that regulate cell growth, survival, and gene expression (14). Integrins regulate diverse pathways including activation of protein tyrosine and serine/threonine kinases, lipid kinases, and small GTPases (15, 16). With regard to survival, some cell-type specificity has been noted. In epithelial and endothelial cells, integrin ligation regulates cell survival such that detachment from the ECM rapidly induces apoptosis (17, 18). In some of these cases, cell death results from increased levels of p53 or Bax caused by detachment or inhibition of integrins or by overexpression of unligated integrins (19–21). Correspondingly, binding of some carcinomas to the ECM protects against apoptosis initiated by DNA damage (22). However, in transformed epithelial cells and fibroblastic cell types, ligation of integrins is not required for survival, and detachment from the ECM is not sufficient to trigger apoptosis (23).
We now show that in some melanoma, sarcoma, and fibroblastic cell types that survive in suspension, detachment from the ECM unexpectedly decreases cell death after DNA damage. The mechanism involves integrin-dependent changes in p14/p19 Arf and p53 levels. Consistent with decreased p53, loss of cell adhesion also induces genetic instability. These results suggest a mechanism by which tumor cells can escape radiation or chemotherapy, acquire additional mutations, and progress toward more aggressive forms.
Materials and Methods
Cells.
HT1080 fibrosarcoma cells were maintained in DMEM supplemented with 10% FCS, nonessential amino acids, and Hepes-buffered saline. M21L melanoma, RD rhabdomyosarcoma, and A375 melanoma cells were maintained in DMEM supplemented with 10% FCS. Mouse embryo fibroblasts (MEFs), p53−/− or p53+/+ cells, and Mdm2−/− or Mdm2+/+ cells also were maintained in DMEM supplemented with 10% FCS. Cells detached from the substratum were resuspended in either standard medium for short times (<1 h) or medium containing 0.8% carboxymethylcellulose for longer times to inhibit cell aggregation. To induce apoptosis, cells were treated with 1–20 μM of the DNA-damaging reagent 5-arabinofuranosylcytosine (ara C) for 24–48 h. Alternatively, cells were irradiated by using a Gammacell 1,000 cesium source. Terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) assays were performed by using the labeling kit from Roche Molecular Biochemicals according to manufacturer instructions. Cells were scored by using fluorescence microscopy.
Antibody Inhibition.
HT1080 fibrosarcoma cells were either kept adherent or detached and resuspended in methylcellulose. At 2 h, ara C was added to some samples. At 2.5 h, 0.5 μg/ml of the purified antiintegrin antibodies P5D2 (Developmental Studies Hybridoma Bank, University of IA, Iowa City), LM534 or LM609 (both kindly provided by D. Cheresh, Scripps Research Institute), or the control antibody 2D3 against CD47 integrin-associated protein (IAP), a gift of E. Brown (Washington University School of Medicine, St. Louis) were added to samples. The addition of antibodies was repeated each hour for a total of 4 doses. After 48 h, lysates were prepared, and caspase-3 activity was measured.
Apoptosis Assays.
For analysis of caspase-3 activation, cell lysates were prepared in 50 mM Hepes, pH 7.4 containing 0.1% CHAPS {3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate}, 0.1 mM EDTA, and 1 mM DTT. Samples were assayed in lysis buffer supplemented with 100 mM NaCl and 10% glycerol using the colorimetric substrate Asp-Glu-Val-Asp (DEVD)-p-nitroanilide with or without the caspase-3 inhibitor Ac-DEVD-CHO (Alexis Biochemicals, San Diego). Background activity (in the presence of inhibitor) was subtracted to obtain specific caspase-3 activity.
Western Blotting.
Cells were lysed in immunoprecipitation assay buffer containing protease inhibitors as described (24). Equal amounts of protein were separated by SDS/PAGE, transferred to nitrocellulose, and developed with antibodies as indicated. Blots were visualized with ECL (Amersham Pharmacia).
Cell Transfections.
Mdm2−/− or Mdm2+/+ MEFs on a p53−/− background were transfected with green fluorescent protein (GFP)-p53 using Effectene according to manufacturer instructions (Qiagen, Chatsworth, CA). Cells were put into suspension at 24 h posttransfection and harvested at the times indicated in the figures. MEFs in 10-cm dishes were transfected with GFP-p53 or GFP-Arf (0.5 μg) plus GFP-GTPase-activating protein (GAP, 1 μg) as a marker for transfection. Cells were put into suspension at 24 h and analyzed for apoptosis at 48 h. The pEGFP-N1 vector expressing GFP-p53 was a generous gift from G. Wahl (Salk Institute, La Jolla, CA). A vector expressing the plasma membrane-targeting protein, GFP-GAP, was kindly provided by A. Horwitz (University of Virginia, Charlottesville, VA). A vector expressing GFP-Arf was a gift of C. Scherr (St. Jude Children's Research Hospital, Memphis, TN).
Karyotype Analysis.
MEFs subjected to 7.5 Gy of gamma irradiation were either replated or kept in suspension for 48 h and allowed to recover. Surviving cells were cultured and expanded for 2–4 weeks. After incorporation of BrdUrd (Sigma), cells were treated with colchicine (KaryoMax, GIBCO/BRL) to arrest cells in mitosis. Metaphases from adherent or suspended cell samples were prepared as described (25). Fixed samples were labeled with anti-BrdUrd antibodies (Sigma) and propidium iodide. Individual metaphases were photographed. Photographic files were shuffled and scored blind for the presence of structural chromosomal aberrations as described by Savage (26).
Results
Cell Adhesion Potentiates Apoptosis in Response to DNA Damage.
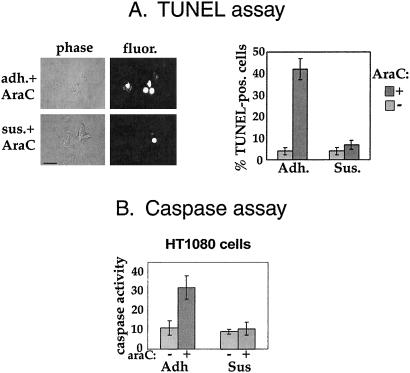
We wished to investigate whether integrin ligation can influence the apoptotic response to DNA damage for tumor cells that survive in suspension. We began by analyzing the response of HT1080 fibrosarcoma cells to DNA damage. We used the drug ara C to induce single- and double-strand breaks and assayed apoptosis in adherent and detached cells. Whereas adherent HT1080 cells showed substantial levels of apoptosis in response to a low dose of ara C as assayed by the TUNEL reaction, cells maintained in suspension for 2 h before ara C exposure showed minimal cell death (Fig. 1A). Additionally, by 3–4 days after treatment nearly 100% of adherent cells were morphologically apoptotic, whereas suspended cells could be replated and remained viable for at least 5 days (data not shown). These data also show that cell detachment confers resistance to DNA damage, not merely a delay in onset of apoptosis. Activity of caspase-3, the executioner caspase, was measured as an independent assay for apoptosis (Fig. 1B). ara C triggered an increase in caspase-3 activity in adherent but not suspended cells correlating with the increased percentage of TUNEL-labeled cells.
Figure 1.
Suspended cells are resistant to apoptosis caused by DNA damage. (A) TUNEL labeling was used to quantitate apoptosis. HT1080 fibrosarcoma cells were either adherent (adh) or detached and resuspended in medium containing methylcellulose (sus). After 2 h, cells were either untreated or treated with ara C. After incubation for 2 days, the percentage of apoptotic cells was determined by using TUNEL labeling (Right). (Left) Phase-contrast images of cells treated with ara C while adherent or suspended; the fluorescence images depict TUNEL labeling of the same cells. Values are means ± SE; n = 4. (Bar, 50 μm.) (B) Caspase-3 activation was used to quantitate apoptosis. HT1080 fibrosarcoma cells were kept adherent or were detached and resuspended in methylcellulose. Cells were either untreated or treated with ara C. Cells were harvested after 48 h, lysates were prepared, and caspase-3 activity was measured. Values are means ± SE; n = 4.
Cell-Type Specificity.
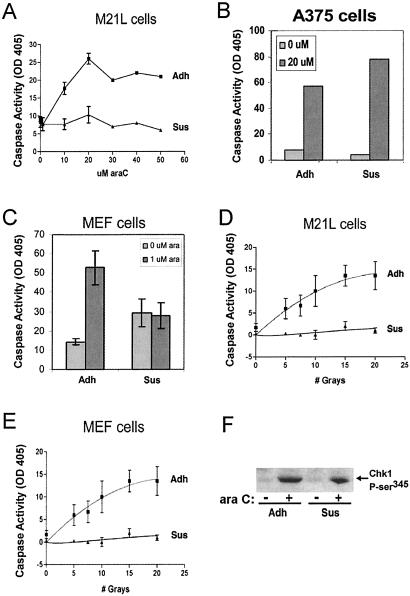
To investigate the generality of these results we examined several other cell types. Although different cell types showed some variation in dose-response curves to ara C (data not shown), M21L human melanoma cells showed a similar adhesion dependence for ara C-induced apoptosis (Fig. 2A), as did human rhabdomyosarcoma cells (data not shown). By contrast, the A375 human melanoma line activated caspase-3 in both adherent and suspended cells after ara C treatment (Fig. 2B). Other cell types examined were not amenable to analysis, because they either were resistant to ara C treatment or the untreated cells died in suspension without ara C. Several normal or carcinoma cell lines including MCF10A epithelial cells and MCF7 breast, A431 bladder, and Du145 prostate carcinomas fell into the latter category. Finally, we tested primary MEFs, because their normal diploid chromosomal structure and the availability of cells from knockout mice could facilitate further studies. MEFs also showed diminished sensitivity to araC when nonadherent (Fig. 2C). These data suggest that the ability of cell detachment to decrease sensitivity to DNA damage may be relatively common among cells that can survive without adhesion but that it clearly shows some cell-type specificity.
Figure 2.
Cell-type specificity and response to ionizing radiation. (A–C) For treatment with ara C, M21L melanoma, A375 melanoma, and MEF cells were kept adherent (Adh) or were detached and resuspended (Sus) in methylcellulose. At 2 h, ara C was added at the indicated concentrations. After 24–48 h, cell lysates were prepared, and caspase-3 activity was measured. Values are means from at least three separate experiments ± SE. (D and E) For gamma irradiation, all cells were detached and irradiated in suspension. Adherent cell samples were replated within 30 min of treatment and incubated for 24–48 h. Suspended cell samples were kept in methylcellulose for 24–48 h. Cell lysates were prepared, and caspase-3 activity was measured. Values are means ± SE; n = 3. (F) MEFs were either kept adherent or detached and resuspended in methylcellulose. At 2 h, ara C was added to some samples. After 4 h in suspension, lysates were prepared, and samples containing equal protein were immunoblotted by using a polyclonal antibody against phospho-serine 345 Chk1 (Cell Signaling Technology, Beverly, MA).
We also tested gamma irradiation as a source of DNA damage. In these experiments, all cells by technical necessity were treated in suspension, which further equalized the conditions of treatment. Adherent cell samples were replated immediately after treatment. Adherent but not suspended M21L cells (Fig. 2D) were sensitive to irradiation, as were MEFs (Fig. 2E) and HT1080 cells (data not shown).
Controls.
Despite the difference in cell death, adherent and suspended cells showed no difference in activation of Chk1, indicating that neither DNA damage itself nor the early response to DNA damage were affected (Fig. 2F). Furthermore, when cells were treated with ara C immediately after detachment from the substratum, they still underwent apoptosis in response to DNA damage (data not shown). Conversely, suspended cells replated immediately after treatment with ara C showed high levels of apoptosis (data not shown but see Fig. 2 D and E for a similar experiment using gamma radiation). These results exclude a number of potential artifacts (for example, decreased uptake of ara C in suspended cells) and suggest that there are temporal effects on adhesion-dependent pathways after changes in adhesion status. The results also show that these effects are reversible.
Role of Integrins.
To investigate the involvement of integrins, various antibodies were tested for their ability to restore the apoptosis response in suspended HT1080 fibrosarcoma cells (Fig. 3). An antibody against the Ig family protein CD47 (IAP, ref. 27) was used as a control. Both anti-IAP and antiintegrin IgGs appeared to cause a slight increase in caspase-3 activation in suspended cells even in the absence of ara C. Treatment of suspended cells with the anti-β1 integrin antibodies LM534 and P5D2 or the anti-αvβ3 antibody LM609 restored sensitivity to ara C, whereas the control IgG had no effect. These data are consistent with studies showing that ligation or crosslinking of integrins with soluble IgGs activate integrin-signaling pathways (28, 29) and demonstrate that integrins mediate these effects.
Figure 3.
Integrins mediate the response to DNA damage. (A) HT1080 fibrosarcoma cells were either kept adherent (Adh) or detached and resuspended in methylcellulose. ara C was added to some samples followed by the addition of antibodies P5D2 (anti-β1), LM534 (anti-β1#), or LM609 (anti-αvβ3) or of the control antibody 2D3 (anti-IAP). After 48 h, lysates were prepared, and caspase-3 activity was measured. Values are means ± SE; n = 3 separate experiments. (B) HT1080 fibrosarcoma cells were detached and resuspended in methylcellulose. At 2.5 h, samples were either untreated (−) or treated with the antiintegrin antibody LM609 (+). Addition of the antibody was repeated each hour for a total of three doses. After 4 h in suspension, lysates were prepared, and samples were immunoblotted by using a polyclonal antibody against Arf (Santa Cruz Biotechnology); antibody alone did not comigrate with Arf on blots. Blots were probed also with anti-ERK2 (Santa Cruz Biotechnology) to demonstrate equal cell protein.
Arf and p53 Levels Decline Rapidly When Cells Are Kept in Suspension.
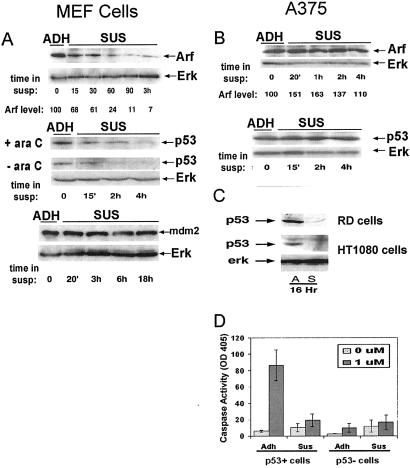
p53 and its upstream regulators p14/19 Arf and Mdm2 comprise a major regulatory pathway for the DNA-damage response in mammalian cells. We therefore investigated the effects of cell adhesion on these components. After detachment of MEFs, Arf protein levels declined dramatically, showing changes within 15 min (Fig. 4A). Levels of Arf continued to decline such that by 90 min Arf was ≈10% of levels in adherent cells and remained low for at least 3 h. An antiintegrin antibody partially restored Arf levels in suspended cells (Fig. 3B). The recovery of Arf was not complete, most likely reflecting the limitations of soluble integrin antibodies that tend to induce only transient signals (30), but was likely to be sufficiently above threshold levels. Notably, Arf in suspended A375 cells, which remain sensitive to DNA damage, did not decline and even showed a modest increase (Fig. 4B). Sensitivity to DNA damage and the integrin regulation of Arf therefore are correlated.
Figure 4.
Role of Arf and p53. MEFs (A and D), human A375 melanoma cells (B), or human HT1080 fibrosarcoma cells and human RD rhabdomyosarcoma cells (C) were detached and either replated (ADH) or maintained suspended (SUS) in methylcellulose. Where indicated, ara C was added. At the indicated times, cell lysates were prepared and analyzed by immunoblotting with polyclonal anti-Arf, monoclonal anti-p53 (clone 421), or monoclonal antibody SMP14 against Mdm2. To demonstrate equal protein loading, blots were reprobed with anti-ERK2. Values for Arf and p53 were normalized to the amount of ERK2 in each lane and are expressed relative to adherent cells at each time. Results are representative of more than three separate experiments. (D) MEFs from a normal mouse (p53+) or a mouse lacking p53 (p53−) were kept adherent or were detached and resuspended in methylcellulose. At 2 h, ara C was added. After 24–48 h, cell lysates were prepared, and caspase-3 activity was measured. Values represent the averages of three separate experiments ± SE.
Analysis of p53 showed that after detachment, p53 protein levels declined with a slower time course (Fig. 4A). Levels of p53 in suspended MEFs remained low for up to 18 h (data not shown). Suspended cells treated with ara C initially increased p53, but levels still declined after 4 h in suspension. This rate correlates with the time required for cells to become resistant to DNA damage after detachment as described above. In HT1080 fibrosarcoma and RD rhabdomyosarcoma cells, p53 was also much lower after detachment (Fig. 4C; HT1080 cells have wild-type p53, and RD cells have p53 mutated in the DNA-binding region; refs. 31 and 32). By contrast, no changes in the level of Mdm2 were observed (Fig. 4A). As expected, p53−/− MEFs are highly resistant to cell death triggered by ara C (Fig. 4D). Together with the result that p53 remains high in suspended A375 cells (Fig. 4B), these results support the hypothesis that the decrease in p53 may account for the diminished sensitivity of suspended cells.
Effects of Adhesion Require Mdm2.
These data suggest that decreased Arf in suspended cells may increase Mdm2-dependent degradation of p53. To test this idea, a GFP-p53 construct in which expression is driven by a constitutive promoter was transfected into p53-null MEF cells. The function and posttranslational regulation of this construct is similar to wild-type p53 (5). The exogenous GFP-p53 protein also declined after cell detachment, whereas levels of transfected control GFP did not (Fig. 5A). This result excludes transcriptional mechanisms for the adhesion-dependent changes in p53 levels. As a further test, we examined MEFs nullizygous for Mdm2. These cells by necessity are also p53-null, hence were transiently transfected with GFP-p53. Lower levels of GFP-p53 DNA were used for these cells to obtain protein levels that did not induce apoptosis without DNA damage. We observed that after 18 h, the decline in p53 in suspended Mdm2-null cells was much less than in Mdm2-positive MEFs (Fig. 5A Bottom). Taken together, these findings argue that the decrease in p53 in nonadherent cells is due at least in part to the Arf/Mdm2 degradation pathway.
Figure 5.
Arf and p53 levels mediate effects on DNA damage response. (A) Wild-type MEFs (Top) or p53-null Mdm2+/+ or Mdm2−/− MEFs (Middle) transiently transfected with GFP-p53 or GFP alone were suspended (Sus) in methylcellulose. At the indicated times, cell lysates were prepared and analyzed by immunoblotting with monoclonal anti-GFP. Blots were reprobed with anti-ERK2 to demonstrate equal loading. The graph (Bottom) compares levels of GFP-p53 remaining in Mdm2+/+ cells or Mdm2−/− cells after 18 h in suspension. Values are means ± SE (n = 3) relative to matched adherent (Adh) cell controls. (B) MEFs were either transfected with GFP-p53 plus GFP-GAP or GFP-GAP alone as a control. The GFP-GAP was included to identify transfected cells. At 24 h posttransfection, cells were either left adherent or detached and put into suspension in methylcellulose. After 2 additional hours, ara C was added where indicated. All cells were incubated for an additional 24 h, and TUNEL assays were performed and scored by fluorescence microscopy. Values are means ± SE (n = 3) and represent the percentage of transfected cells positive for the TUNEL label. (C) MEFs were either transfected with GFP-Arf plus GFP-GAP or GFP-GAP alone as a control. The GFP-GAP was included to identify transfected cells. At 24 h posttransfection, cells were either left adherent or detached and put into suspension in methylcellulose. After 2 h, ara C was added where indicated. All cells were incubated for an additional 24 h, and TUNEL assays were performed and scored by fluorescence microscopy. Values are means ± SE (n = 3) and represent the percentage of transfected cells positive for the TUNEL label. (D) MEFs transiently transfected as described above were analyzed by Western blotting for p53 and for Erk as a control for total cell protein. Levels were quantified by densitometry, and p53 was normalized for Erk within each sample. Values are means ± SD relative to adherent GFP-GAP cells for each experiment (n = 5).
Restoration of p53.
To test the functional relationship between p53, Arf, and apoptosis, MEF cells were transfected with p53 or Arf at moderate levels that cause only slight apoptosis in the absence of DNA damage. As a marker of transfection, cells were cotransfected with a plasma membrane-targeted GFP construct, GFP-GAP (33). Cells were either untreated or treated with ara C. We observed that elevating p53 or Arf increased sensitivity to ara C in suspended cells nearly to the level found in adherent cells (Fig. 5 B and C). Although there was some elevation of apoptosis in the suspended GFP-GAP control cells in the Arf reconstitution experiments, statistical analysis showed that the increase in ara C killing in suspended Arf-transfected cells relative to suspended GFP-GAP cells is highly significant (P = 0.007).
To confirm that p19Arf expression increased p53 levels, transiently transfected cells were assayed by Western blotting. In cells transfected with the GFP-GAP control alone, p53 in suspended cells declined to 24% of adherent cells (Fig. 5D). These levels of p53 are slightly higher than untransfected cells, probably indicative of stress caused by transfection. Expression of GFP-Arf caused an increase in p53 in adherent cells (64% above their GFP-GAP counterparts) and substantially inhibited the decline in suspended cells (59% of adherent p19Arf-transfected cells). Given the transfection efficiencies of ≈50%, these results indicate that p19Arf expression strongly inhibits the decline in p53 after detachment. Thus, raising levels of p53 or Arf in suspended cells restored the response to DNA damage. We also noted some apoptosis without ara C in adherent but not suspended GFP-p53-expressing cells; this result is consistent with the higher level of GFP-p53 in the adherent cells (Fig. 5A).
Chromosomal Instability.
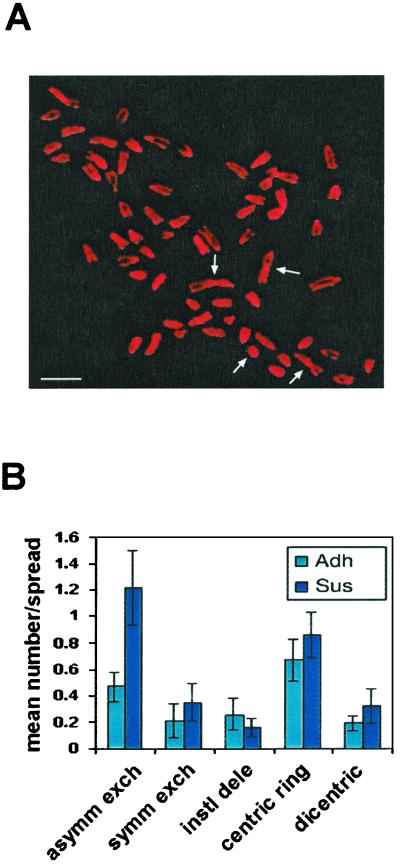
p53 is critical for maintaining integrity of the genome (34, 35), and hence decreased p53 in suspended cells could cause genetic instability. Treatment with radiation or chemotherapy then could lead to accumulation of mutations at an elevated rate. As an initial test of this hypothesis, irradiated MEFs were subjected to karyotype analysis. As described before, cells were irradiated while in suspension but then either replated immediately to restore p53 function or maintained in suspension for 2 days before replating. The irradiation caused most of the cells to die; the few surviving cells then were expanded and analyzed. As expected, both irradiated samples contained cells with aberrant chromosomes (Fig. 6). However, cells kept in suspension contained a significantly larger number of gross chromosomal rearrangements. These data provide evidence that loss of integrin-mediated attachment during times of genotoxic stress may lead to the growth of cells harboring chromosomal rearrangements.
Figure 6.
Effect of adhesion on radiation-induced chromosomal rearrangements. Wild-type MEFs were detached and subjected to 7.5 Gy of gamma irradiation. Adherent (Adh) cell samples were replated within 30 min; suspended cell samples (Sus) were kept in methylcellulose for 24 h and then replated, and all cells were cultured for 2–4 weeks. Karyotype analysis was performed to assess gross chromosomal rearrangements. (A) A representative metaphase spread from cells irradiated in suspension; several chromosomal aberrations are visible in the photograph and are indicated by arrows. (B) Spreads were scored for the presence of aberrations as shown; values are means ± SE from 40 metaphases. asymm exch, asymmetric exchanges; symm exch, symmetric exchanges; instl dele, interstitial deletion. (Bar, 10 μm.)
Discussion
These results demonstrate that for several cell types in which survival per se does not require adhesion, integrin-mediated cell adhesion positively regulates the DNA-damage response such that cells maintained in suspension show less apoptosis in response to either radiation or a radiomimetic chemical. The effect is caused by changes first in levels of p19Arf and subsequently in levels of p53 tumor suppressor. Consistent with the reduction in p53, suspended cells also show elevated mutation rates after irradiation. The results presented here suggest that a rapid decrease in Arf levels after cell detachment leads to decreased p53 levels, consistent with the known ability of Arf to suppress MDM2 and prevent p53 degradation (4, 36, 37). Loss of p53 then mediates the decreased sensitivity to DNA damage. Under some conditions, Arf has been shown to act independently of p53 to cause cell cycle arrest in G1 (38, 39). Thus, loss of Arf also may have effects independent of p53 that may alter the DNA-damage response. In addition, c-Abl is both regulated by integrins and involved in the DNA-damage pathway (40, 41) and thus also could contribute to the adhesion-sensitive DNA-damage response.
There have been many studies investigating how loss of integrin-mediated adhesion promotes apoptosis in epithelial and endothelial cell types, whereas other cell types including fibroblasts are much less sensitive to detachment. Only under conditions of severe growth-factor deprivation do fibroblastic cells show effects of specific integrins on survival (42). The mechanisms that account for cell-type specificity are unknown, and it is unclear to what extent integrin protection from cell death in epithelial/endothelial cells occurs by the same mechanisms as those in less sensitive cell types.
We noted that the carcinoma and epithelial cell lines we tested became apoptotic when detached in the absence of DNA damage. In these cells, there is some evidence for pathways linking integrin α6β4 to activation of p53. Bachelder et al. (21) showed that overexpression of α6β4 integrin in suspended carcinoma cells, where the integrin would be unoccupied by any ECM ligand, activates p53 and induces apoptosis. They also reported that antibody crosslinking of unoccupied integrins accelerated apoptosis; however, this effect is unlikely to reflect interactions of α6β4 with basement membranes, because adhesion to basement membranes promotes epithelial cell survival. p53 also was linked to survival of adherent rabbit synovial fibroblasts or mouse endothelial cells induced by growth-factor deprivation. In that study, focal adhesion kinase-mediated decreased p53 levels and prevented apoptosis (43). Because integrin α6β4 does not activate focal adhesion kinase, it seems unlikely that these two effects are related, despite the involvement of p53 in both. Furthermore, both of these effects of integrins on p53-dependent apoptosis are quite opposite to those described in our study. Given the opposite direction of the effects, the different conditions and cell types examined, and the large number of pathways known to activate p53, there are no compelling reasons to believe that these distinct effects are related.
The pathways by which integrin-mediated adhesion regulates Arf levels are unknown currently. Integrins have been shown to stimulate the classical mitogen-activated protein kinase pathway (44–46), which when strongly activated by oncogenic Ras can increase both Arf and Mdm2 levels (47). However, the mitogen-activated protein kinase kinase inhibitor PD98059 had no effects on Arf in MEF cells (data not shown and ref. 48), arguing against a role for Erk in this system. Arf expression is induced also by death-associated protein kinase and the transcription factors DMP1 and E2F1 after activation of the oncogenes c-myc and c-ras; it is opposed by Twist and Bmi1 (49). Because integrins control the activity or levels of several of these components (50, 51), there are a number of candidate pathways that might mediate the effects of adhesion on Arf.
Our findings provide a mechanistic link between cell adhesion and DNA damage-induced apoptosis and may have implications for models of tumor cell progression. First, tumor cells in vivo that are poorly adherent may escape killing induced by DNA-damage therapy. Second, such cells may show enhanced accumulation of mutations after DNA damage, which would thereby accelerate progression. Finally, our data might offer a new strategy for therapy, in which ligating integrins or stimulating the relevant integrin pathway may be effective as an adjunct to conventional radiation and chemotherapies to enhance killing of poorly adherent tumor cells.
Acknowledgments
We thank Dr. Johann Lin for instruction in karyotype analysis techniques. We are grateful to Dr. G. Lozano (Baylor University) for generously providing Mdm2−/− cells. We thank Dr. Geoff Wahl and A. F. Horwitz for GFP-p53 and GFP-GAP, respectively. We also thank Dr. Charles Sherr for the generous gift of GFP-Arf. Finally, we thank Drs. Mark Ginsberg, Sanford Shattil, and Natalie Marachenko for their comments and suggestions. This study was supported by U.S. Public Health Service National Institutes of Health Grants R29 CA74230 (to J.M.L.) and P01 HL48728 (to M.A.S.).
Abbreviations
- ECM
extracellular matrix
- MEF
mouse embryo fibroblast
- ara C
5-arabinofuranosylcytosine
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
- IAP
integrin-associated protein
- GFP
green fluorescent protein
- GAP
GTPase-activating protein
References
- 1.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 2.Oren M. Semin Cancer Biol. 1994;5:221–227. [PubMed] [Google Scholar]
- 3.Ko L J, Prives C. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 4.Tao W, Levine A J. Proc Natl Acad Sci USA. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stommel J M, Marchenko N D, Jimenez G S, Moll U M, Hope T J, Wahl G M. EMBO J. 1999;18:1660–1672. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L C, Clarkin K C, Wahl G M. Proc Natl Acad Sci USA. 1996;93:4827–4832. doi: 10.1073/pnas.93.10.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirao A, Kong Y Y, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge S J, Mak T W. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 8.Prives C. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- 9.Amundson S A, Myers T G, Fornace A J., Jr Oncogene. 1998;17:3287–99. doi: 10.1038/sj.onc.1202576. [DOI] [PubMed] [Google Scholar]
- 10.Miyashita T, Reed J C. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 11.Oda K, Arakawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y, Taya Y. Cell. 2000;102:849–862. doi: 10.1016/s0092-8674(00)00073-8. [DOI] [PubMed] [Google Scholar]
- 12.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 13.Kuerbitz S J, Plunkett B S, Walsh W V, Kastan M B. Proc Natl Acad Sci USA. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assoian R K. J Cell Biol. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 16.Giancotti F G, Ruoslahti E. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 17.Meredith J E, Jr, Fazeli B, Schwartz M A. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisch S M, Francis H. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore A P, Metcalfe A D, Romer L H, Streuli C H. J Cell Biol. 2000;149:431–446. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stromblad S, Becker J C, Yebra M, Brooks P C, Cheresh D A. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachelder R E, Marchetti A, Falcioni R, Soddu S, Mercurio A M. J Biol Chem. 1999;274:20733–20737. doi: 10.1074/jbc.274.29.20733. [DOI] [PubMed] [Google Scholar]
- 22.Sethi T, Rintoul R C, Moore S M, MacKinnon A C, Salter D, Choo C, Chilvers E R, Dransfield I, Donnelly S C, Strieter R, Haslett C. Nat Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 23.Meredith J E, Jr, Winitz S, Lewis J M, Hess S, Ren X D, Renshaw M W, Schwartz M A. Endocr Rev. 1996;17:207–220. doi: 10.1210/edrv-17-3-207. [DOI] [PubMed] [Google Scholar]
- 24.Lewis J M, Cheresh D A, Schwartz M A. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey S M, Meyne J, Chen D J, Kurimasa A, Li G C, Lehnert B E, Goodwin E H. Proc Natl Acad Sci USA. 1999;96:14899–14904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savage J K R. J Med Genet. 1975;12:103–122. [Google Scholar]
- 27.Lindberg F P, Gresham H D, Schwarz E, Brown E J. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis J M, Schwartz M A. Mol Biol Cell. 1995;6:151–160. doi: 10.1091/mbc.6.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz M A, Ingber D E, Lawrence M, Springer T A, Lechene C. Exp Cell Res. 1991;195:533–535. doi: 10.1016/0014-4827(91)90407-l. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz M A, Lechene C, Ingber D E. Proc Natl Acad Sci USA. 1991;88:7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slebos R J, Taylor J A. Biochem Biophys Res Commun. 2001;281:212–219. doi: 10.1006/bbrc.2001.4335. [DOI] [PubMed] [Google Scholar]
- 32.Felix C A, Kappel C C, Mitsudomi T, Nau M M, Tsokos M, Crouch G D, Nisen P D, Winick N J, Helman L J. Cancer Res. 1992;52:2243–2247. [PubMed] [Google Scholar]
- 33.Moriyoshi K, Richards L J, Akazawa C, O'Leary D D M, Nakanishi S. Neuron. 1996;16:255–260. doi: 10.1016/s0896-6273(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 34.Honma M, Momose M, Tanabe H, Sakamoto H, Yu Y, Little J B, Sofuni T, Hayashi M. Mol Carcinog. 2000;24:203–214. doi: 10.1002/1098-2744(200008)28:4<203::aid-mc3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Venkatachalam S, Shi Y P, Jones S N, Vogel H, Bradley A, Pinkel D, Donehower L A. EMBO J. 1998;17:4657–4667. doi: 10.1093/emboj/17.16.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haupt Y, Maya R, Kazaz A, Oren M. Nature (London) 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 37.Kubbutat M H, Jones S N, Vousden K H. Nature (London) 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 38.de Stanchina E, McCurrach M E, Zindy F, Shieh S Y, Ferbeyre G, Samuelson A V, Prives C, Roussel M F, Sherr C J, Lowe S W. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber J D, Jeffers J R, Rehg J E, Randle D H, Lozano G, Roussel M F, Sherr C J, Zambetti G P. Genes Dev. 2000;14:2358–2365. doi: 10.1101/gad.827300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis J M, Baskaran R, Taagepera S, Schwartz M A, Wang J Y. Proc Natl Acad Sci USA. 1996;93:15174–15179. doi: 10.1073/pnas.93.26.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J Y. Oncogene. 2000;19:5643–5650. doi: 10.1038/sj.onc.1203878. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Vuori K, Reed J C, Ruoslahti E. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ilic D, Almeida E A, Schlaepfer D D, Dazin P, Aizawa S, Damsky C H. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q, Kinch M S, Lin T H, Burridge K, Juliano R L. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 45.Renshaw M W, Ren X D, Schwartz M A. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlaepfer D D, Hanks S K, Hunter T, van der Geer P. Nature (London) 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 47.Ries S, Biederer C, Woods D, Shifman O, Shirasawa S, Sasazuki T, McMahon M, Oren M, McCormick F. Cell. 2000;103:321–330. doi: 10.1016/s0092-8674(00)00123-9. [DOI] [PubMed] [Google Scholar]
- 48.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vousden K H. Cell. 2000;103:691–694. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 50.Cary L A, Han D C, Guan J L. Histol Histopathol. 1999;14:1001–1009. doi: 10.14670/HH-14.1001. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz M A, Baron V. Curr Opin Cell Biol. 1999;11:197–202. doi: 10.1016/s0955-0674(99)80026-x. [DOI] [PubMed] [Google Scholar]