Abstract
This Review explores the status and progress made over the past decade in the areas of raw material mining, battery materials and components scale-up, processing, and manufacturing. While substantial advancements have been achieved in understanding battery materials, the transition to large-scale manufacturing introduces scientific challenges that must be addressed from multiple perspectives. Rather than focusing on new material discoveries or incremental performance improvements, this Review focuses on the critical issues that arise in battery manufacturing and highlights the importance of cost-oriented fundamental research to bridge the knowledge gap between fundamental research and industrial production. Challenges and opportunities in integrating machine learning (ML) and artificial intelligence (AI) to digitalize the manufacturing process and eventually realize fully autonomous production are discussed. The review also emphasizes the pressing need for workforce development to meet the growing demands of the battery industry. Potential strategies are suggested for accelerating the manufacturing of current and future battery technologies, ensuring that the workforce is equipped with the necessary skills to support research, development, and large-scale production.

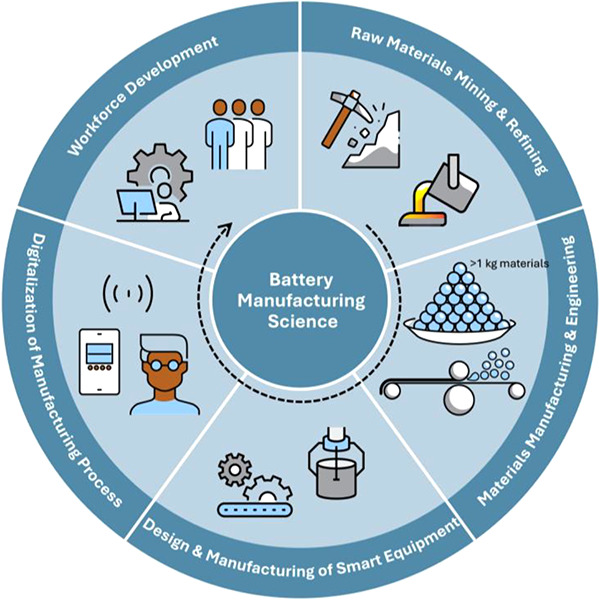
1. Introduction
In light of the escalating importance of batteries for broad applications in modern societies, it is crucial to establish a sustainable strategy that pinpoints and addresses the scientific challenges in battery manufacturing. For academic researchers, manufacturing seems a little far from their daily research, as they are not familiar with the manufacturing process which is usually taken care of by industry. It is not clear what kind of roles researchers can play in supporting battery manufacturing. Meanwhile, for industry, most of the published research is valuable in deepening scientific understanding, but there is still uncertainty whether those new materials or ideas are suitable for large-scale production, because the first criterion to assess the feasibility of any new material, component, or battery technology is the cost, which includes both materials and processing.
The cost of Li-ion batteries (LIBs) has dropped significantly from a few thousand dollars per kWh in the 1990s to around $100/kWh today. However, to further accelerate electric vehicle (EV) market penetration and enable large-scale deployment of grid energy storage, the cost must be reduced below $100/kWh. Achieving this requires cost-efficient materials and processing technologies along with increased cell-level energy density, because the cost of a battery is defined as $$/kWh. In addition to the cell-level energy from each building block of the pack, battery lifetimethat is, the total deliverable energy during the lifespan of the batteryalso plays a key role in determining the cost in the long term, which needs more fundamental understanding about stabilizing the structures of cathodes and their interfaces, which is not the focus of this Review. CO2 emission from battery manufacturing has been covered by quite a few review papers − and thus will not be discussed herein.
Fundamental research related to battery manufacturing needs to be cost oriented. For conventional LIBs, graphite production is quite mature. To further reduce the cost of graphite production, natural graphite is of great importance, but its electrochemical performance still needs further improvement without introducing an additional cost. The manufacturing of high-performance graphite also relies on equipment such as specialized furnaces to precisely tune the temperatures and heating environments to control the electrochemical properties of carbon. Hard carbon is also proposed for LIBs and, so far, is the best anode material for Na-ion batteries. Synthesis of hard carbon from sustainable resources is one of the promising directions from a manufacturing cost and scalability point of view.
For the conventional LiNi0.6Mn0.2Co0.2O2 (NMC622) cathode, the manufacturing costs fluctuate with raw material prices, including lithium salts and transition metal sulfates. Additionally, various synthesis approaches also impact the production cost of NMC materials. New approaches are needed to increase the tolerance level of raw materials while simplifying the manufacturing process of NMC cathode materials and reducing the carbon footprint during production. As more nickel is packed into NMCs, e.g., LiNi0.8Mn0.1Co0.1O2 (NMC811) or LiNi0.9Mn0.05Co0.05O2(NMC90), different challenges arise, delaying their large-scale adoption for use in commercial EV batteries. For high-nickel NMCs, the moisture sensitivity, gassing, and cracking issues are still not well addressed; however, they might be overcome through cost-effective synthesis innovations. Similarities can be found in other cathode materials, such as LiFePO4, LiMnPO4, and LiFe1–x Mn x PO4, which still need to be downsized to nanoparticles for sufficient ionic conductivity, but that raises problems in making dense and thick electrodes for high-energy applications. All of these issues are new opportunities to integrate materials science and engineering to tackle the pressing challenges in reducing manufacturing cost for battery materials with enhanced properties and performances.
To manufacture next-generation high-energy batteries such as lithium metal (solid or liquid) or lithium–sulfur batteries, processing costs need to be further lowered through innovations. For example, ideally, if coupled with an NMC cathode, no lithium metal is needed on the anode side, assuming the Li+ stored in the NMC cathode can be 100% reversibly utilized back and forth. However, due to the aggressive side reactions between Li and electrolytes, Li stored in an NMC cathode is quickly consumed, leading to very fast cell degradation. Before an “anode-free” cell is realized, an appropriate amount of Li metal is still necessary on the anode side to compensate for the continuous loss of Li+ ions upon each cycle. The thinner the lithium metal foil, the lower the material cost, but processing ultrathin lithium metal foil below 20 μm could be quite expensive. Another good example is the sulfur cathode. Sulfur is almost “free” compared to NMCs because it is the byproduct of crude oil processing. But sulfur/carbon-based composites still have challenges before their successful implementation in Li-S batteries. For example, the highly porous S/C electrode absorbs at least twice the amount of electrolyte as in conventional NMC cathodes, thus sacrificing cell-level energy. Reducing the porosity and tortuosity of the S/C composite electrode is not easy because of the nature of their nanostructures. Solid-state electrolytes have already achieved high ionic conductivity at room temperature, comparable to that of liquid electrolyte. But processing the particles into solid membranes will result in some loss in conductivity due to the presence of grain boundaries, which may become limiting at practical conditions. In addition, large-format processing of solid-state membranes without any defects is a prerequisite for commercializing solid-state batteries, although most of the demonstrated cells still have limited dimensions. ,
To directly support battery manufacturing, different mindsets are needed in performing fundamental research. It is critical for us to understand the true challenges in lowering the cost of battery materials and processing through a comprehensive survey from the raw materials supply chain to production. Advanced in-line characterization and smart manufacturing will further increase the yields and reduce the cost of “waste”. Scrapes from the production line as well as the spent batteries can be directly recycled and fed back into the manufacturing line as “raw” materials. If the cost to recycle spent batteries becomes comparable to that of the freshly mined materials, then those recycled batteries would also be good “feedstock” to make fresh batteries, reducing the supply chain risks. To ensure sustainable manufacturing, workforce developmentfrom operators to researchersneeds to happen in parallel with tailored courses for various industries, and they need to reach the best practical impacts.
2. Raw Materials Mining and Refining
2.1. Lithium Salts
2.1.1. Lithium Extraction
Raw lithium salts are the building blocks of LIBs from cathode synthesis to electrolyte production and anode prelithiation. Lithium is mainly extracted from natural resources such as rocks, spodumene LiAl(SiO3)2, or brines. There is more lithium stored in continental brines than in hard rock ores. The former is the primary mining method for lithium production.
Extraction of lithium from brines relies on evaporitic technology, which pumps the saltwater from underground aquifers to the surface and relies on open-air evaporation to concentrate the brine. This process takes up to two years, which is very slow and cannot respond resiliently to the market need. The brine solution is then processed by adding chemicals to precipitate and refine lithium salts. , The locations of brines suitable for lithium extraction are geographically restricted. Brine sources with diluted lithium such as geothermal brines cannot adopt the conventional evaporation method for lithium extraction due to their different chemistry and much longer time for processing. However, new extraction methods such as direct lithium extraction, e.g., ion exchange, electrochemical methods, etc. are being investigated which may accelerate the process of concentrating lithium salts in all brines with different lithium concentrations, if scalability can be demonstrated.
In hard rock deposits, lithium is present in the form of Li2O in spodumene, the content of which varies from 2.9 to 7.6 wt%. The mainstream approach to extract lithium from spodumene is through acid roasting (phase transition and sulfuric acid digestion) (Figure ). A high-temperature calcination step is required to first convert the α-type monoclinic structure of spodumene to the β-type tetragonal form, with a selective sieving process to increase Li2O concentration. Then the converted spodumene is mixed with an excessive amount of sulfuric acid, reacting at 250 °C. The related chemical reactions for acid roasting are shown in Figure . Li2O will dissolve in sulfuric acid, forming Li2SO4, which will eventually be converted to insoluble Li2CO3 by using a Na2CO3 solution. It has been found that the leaching rate of the impurities in the spodumene is slow. Adding additional amounts of sulfuric acid does not increase the extraction yield of lithium, indicating that not all the impurities in spodumene are accessible by acid, depending on their specific locations or environment in the hard rock. To further improve the extraction yield, more fundamental research is necessary to quickly understand the distribution of different impurities before and after acid roasting. Energy involved in the extraction of lithium is also intensive. Reducing the temperature needed for phase transition of spodumene, shortening the time used for high-temperature calcination by energy-efficient use of fluidized-bed systems, or decreasing the amount of strong acid used are all important research areas to improve lithium extraction from rocks.
1.
A simplified process diagram for lithium carbonate production with lithium brine extraction and hard rock mining. Modified and reproduced from ref under the terms of the CC-BY 4.0 license.
Concentrated lithium, extracted from either brines or hard rocks, will undergo a series of refining process to meet battery-grade purity (Figure ), removing major impurities such as Mg and Ca by using chemical agents such as Ca(OH)2 and Na2CO3, respectively, which counts for 30–40% of the total cost of lithium production and is accompanied by CO2 emission. In the refinery process, Mg removal is still much more challenging than Ca removal because of Mg’s size being almost identical with that of Li. The presence of Mg impurity considerably impacts the extraction efficiency of Li from brines, making it challenging to separate them. An increased Mg/Li mass ratio dramatically decreases the feasibility of extracting Li from the corresponding brines, most of which, unfortunately, have a high Mg/Li ratio. In addition, Mg precipitates into smaller particles compared to their calcium counterpart, not to mention that the concentration of Mg is higher than that of Ca, especially from those brines with high Mg/Li ratios. All of these factors add to the increased cost of eliminating Mg during the refining process. Therefore, new but cost-efficient approaches are urgently needed to expand available brine sources for lithium extraction.
A recent interesting work found that up to 1% Mg impurity in raw lithium salt materials in fact improves the electrochemical performances of cathode materials synthesized using Li2CO3, with this “high” impurity of Mg presumably assigned to the doping effects of Mg. Indeed, Mg is a common dopant used to tune the lattice structure and thus the rate and/or cycling stability of battery materials. , If some of the impurities in the raw lithium salts can be utilized as dopants to positively impact the later-formed functional materials, it will greatly ease and accelerate the production of raw lithium salts and, therefore, reduce the cost of lithium extraction.
The extracted salts are either further utilized to prepare lithium metal ingots through electrolysis, which will be discussed in a later section, or processed to battery-grade-purity salts for battery materials synthesis or electrolyte use.
2.1.2. Salts as Precursors for Battery Materials Synthesis
Li2CO3, LiOH·H2O, and LiOH, extracted and refined from brines or rocks, are the commonly used salts for battery materials production. The synthesis of commercial Ni-rich cathodes such as polycrystal NMC811 often utilizes LiOH or LiOH·H2O as a lithium salt precursor, while low-cost Li2CO3 is used for manufacturing NMC622, NMC532, and LiNi1/3Mn1/3Co1/3O2 (NMC111), which have reduced nickel content.
Li2CO3, LiOH·H2O, and LiOH along with LiNO3 and Li3PO4 are also used for synthesis of phosphate cathodes such as LiFePO4 or LiMnPO4, depending on the specific route used. From a cost point of view, Li2CO3 is still the preferred raw salt for mass production of phosphates cathode materials because, as discussed earlier, Li2CO3 is the most common form of lithium salt after being extracted and processed from rocks or brines. An interesting work took advantage of a lithium salt such as Li1–x Mg y V2–z N z O5 as an additive mixed with preheated LiFePO4 precursors, followed by milling and sintering to form a composite-phase material. The salt additive has both good ionic and electronic conductivities which “glue” nanosized LiFePO4 into micron-sized particles (D50: 2 μm), which not only increases the tap density of as-prepared LiFePO4 but suppresses the particle growth of LiFePO4 during high-temperature calcination, leading to excellent rate capabilities for both charge and discharge.
Current wisdom believes that lithium salts need to be downsized homogeneously to be mixed with different precursors for a uniform chemical reaction to happen during high-temperature calcination. This leads to the necessity of premilling and/or sieving lithium salts, which adds on the cost. If chemical lithiation can happen without the need to control the particle size of lithium salts, the materials manufacturing efficiency will be improved. Impurities such as iron and sodium in the lithium salts need to be cautiously controlled and monitored because they not only impact the structural stability of the as-prepared cathode materials or electrolytes but also may contaminate the anode or damage the solid–electrolyte interphase (SEI) upon cycling.
2.1.3. Salts for Electrolyte
Liquid electrolytes used for batteries are made by dissolving salts in solvents. Dissociated salts in the electrolyte solution provide ionic conductivity, which is dominated by lithium hexafluorophosphate (LiPF6) for LIBs. Some other salts, such as lithium hexafluoroarsenate (LiAsF6), lithium borohydride (LiBH4), and so on, are also used but mainly as additives. Lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium bis(fluoromethanesulfonyl)imide (LiFSI) have corrosion issues at elevated voltages, − but the discovery of concentrated electrolytes enabled their broad application in not only Li-ion but also Li metal batteries.
LiPF6 is manufactured by reacting phosphorus pentafluoride (PF5) with lithium fluoride (LiF) by following the overall reaction below.
From the above reactions, the key raw materials used for LiPF6 production are hydrogen fluoride (HF), LiCl, and phosphorus pentachloride (PCl5). The PCl5 is mixed with HF to prepare PF5 (Reaction 1). Then PF5 reacts with LiCl in liquid HF to form LiPF6 (Reaction 2). Anhydrous HF, obtained by treating liquid HF with high-purity fluorine (F2) gas (from electrolysis), is needed for reactions. High-purity F2 gas is from an electrolysis process.
The whole process is illustrated in Figure . Hazardous HF is always needed for LiPF6 production, raising the manufacturing cost due to safety and control measures. But for now, there is no alternative solvent that works better than HF for LiPF6 production to give the high yield and purity needed in LiPF6 manufacturing.
2.
Process flow for LiPF6 production. Reproduced with permission from ref . Copyright 2019 American Chemical Society.
LiFSI is emerging as a next-generation lithium salt for electrolytes, complementing LiPF6. Following its initial industrialization by Nippon Shokubai, major manufacturers within the lithium battery supply chain in China, Japan, and Korea have commenced the large-scale production of LiFSI and are progressively expanding their production capacities.
The synthesis of LiFSI typically consists of three
primary steps:
(1) preparation of dichlorosulfonimide (Reaction
1 below), (2) fluorination of dichlorosulfonimide (Reaction 2), and (3) lithium ion exchange (Reaction 3). Although
the overall synthesis route for LiFSI is fixed, the raw materials
and process conditions utilized in each step can vary depending on
the specific route employed. The mainstream
synthesis pathway for LiFSI is illustrated in the reactions below.
−

Recent market trends suggest a steady increase in market penetration of LiFSI, with continued growth expected in the foreseeable future. In comparison to LiPF6, LiFSI exhibits substantially enhanced thermal and chemical stability with higher resistance to hydrolysis. Electrolytes based on LiFSI demonstrate improved performance across both high- and low-temperature regimes, as well as superior rate capability, attributed to the higher ionic conductivity and reduced SEI resistance. The development of high-concentration electrolytes (HCEs) based on LiFSI has opened a brand new direction for studying electrode–electrolyte interphases, which has a potential to be applied in certain battery technologies. This enhancement brough by HCEs is primarily attributed to the solvation structure, wherein the Li+ cation exhibits a strong association with FSI– anions as well as the solvent, facilitating the formation of an anion-derived SEI. The resulting SEI is more stable and robust than the conventional solvent-derived SEI, as it is predominantly composed of inorganic components, which exhibit resistance to dissolution and mitigate continuous formation in subsequent cycles.
However, a notable limitation of HCEs is the high concentration of salts, which leads to increased viscosity and higher costs. To mitigate these issues, localized high-concentration electrolytes (LHCEs) have been proposed. LHCEs utilize weak or nonsolvating solvents as diluents, thereby retaining the beneficial properties of HCEs while reducing the overall salt concentration and viscosity to levels akin to those of conventional electrolytes. This strategy helps reduce the cost of LHCEs relative to HCEs by decreasing the salt concentration in the electrolyte. It also improves the cycling stability, calendar life, and operational temperature range of the batteries. −
Yet, the production cost of LiFSI still remains higher than that of LiPF6, primarily due to the more complex synthesis route and the lower yield of LiFSI, whereas LiPF6 has been in production for a longer period and is well-established in the market, benefiting from a more standardized synthesis process. , However, ongoing advancements in the production processes of LiFSI, coupled with potential breakthroughs in industrial-scale manufacturing and accelerated market demands, could lead to cost reductions, positioning LiFSI as a promising candidate for future applications, particularly due to its reduced fluorination compared to LiPF6.
Impurity Impacts
Impurities in electrolytes critically influence the performance and safety of LIBs. LiPF6 commonly contains impurities such as water, hydrofluoric acid (HF), metal contaminants (such as Na, K, Ca, Mg, Fe, Cu), anion contaminants (including Cl– and SO4 2–), and insoluble particulates. Similar impurities are observed in other commercial electrolyte salts, such as LiBF4, LiAsF6, and LiFSI, although additional impurities may exist as well. Besides, impurities also come from electrolyte solvents, usually including water and organic contaminants. These impurities arise from various sources and adversely affect battery performance in different ways. For example, a trace amount of water can infiltrate the electrolyte due to inadequate handling, insufficient drying, or exposure to humid conditions. Water reacts with salts like LiPF6 to generate HF, a highly corrosive byproduct that exacerbates electrolyte degradation over time. −
Metal contaminants in the form of metal particulates or metal ions, commonly originating from contaminated raw materials or equipment, negatively impact the anode performance by inducing metallic deposition. These contaminants also poison the SEI layers and accelerate the degradation of the electrolyte. Cl– and SO4 2– usually stem from contaminated materials and the LiPF6 synthesis processes. These anions accelerate the corrosion of the aluminum current collectors. Additionally, they may react with other components to form insoluble precipitates, thereby increasing the internal resistance of the battery. Organic impurities, such as acids, alcohols, and aldehydes, are mainly residual reactants or byproducts from solvent production processes. They can engage in undesired oxidation/reduction reactions at the electrode surfaces, diminishing battery efficiency. In lithium metal batteries, impurities containing active protons can undergo severe chemical reactions with the lithium metal anode due to the highly reactive nature of metallic lithium. Of note, there is also a report on the water-derived HF “guiding” lithium metal growth into columns, smoothing dendrite growth.
Rigorous monitoring and control of electrolyte impurities are essential to maintain the high quality of the electrolyte and ensure optimal battery performance, reproducibility, and safety. China has established a comprehensive set of chemical industrial standards governing the production and application of common electrolyte materials, including salts, solvents, and additives. − These standards outline requirements for product quality, test methods, and inspection procedures, as well as guidelines for marking, labeling, packaging, transportation, and storage. For instance, the standards stipulate that LiPF6 must meet a minimum purity of 99.95%, whereas commonly utilized carbonate solvents are mandated to achieve a minimum purity of 99.99% for battery application. In academic research, when developing innovative electrolytes that incorporate chemicals beyond conventional salts and solvents, it is crucial for researchers to exercise rigorous quality control during electrolyte preparation. Additionally, detailed information about chemical purity and impurities may need to be disclosed in publications to ensure the reliability and reproducibility of the electrolyte for practical applications.
Electrolyte Recycling
Considering the amount of electrolyte (15–20 wt%) used in batteries and the cost of battery-grade salts, electrolyte recycling is underemphasized in battery recycling research. Early literature on LIB recycling proposed liquid extraction methods for electrolyte recycling, while subsequent studies of battery recycling often overlooked electrolyte recovery. , Considering the high volatility, flammability, and toxicity associated with contemporary battery electrolytes, improper management of these materials in spent batteries poses substantial environmental and health risks. − In addition to the economic benefits of recovering valuable electrolyte components, it is imperative to address these hazards effectively to mitigate potential adverse effects.
However, the electrolyte in spent LIBs is predominantly absorbed within the porous electrode materials, making electrolyte recycling more challenging. To overcome these difficulties and improve the efficiency of the electrolyte recycling process, several approaches have been proposed. The first approach is to recover the electrolyte through a liquid extraction method. Because of the high cost associated with liquid solvent extraction, researchers have explored the use of liquid and supercritical CO2-assisted methods to improve electrolyte recovery rates. − In contrast to traditional liquid solvent extraction techniques, the supercritical CO2-assisted approach facilitates the efficient removal and recovery of the electrolyte through pressure relaxation. High effectiveness in supercritical fluid extraction can be achieved by optimizing operational parameters such as pressure, temperature, and extraction duration. For example, a recovery rate of 89.1 ± 3.4 wt% of the electrolyte from Panasonic 18650 batteries was attained using supercritical CO2 and an acetonitrile/propylene carbonate mixture in a 3:1 ratio. Given that spent electrolytes are composed of a complex mixture of salts, solvents, additives, and their decomposition products, and considering the wide variety of battery types and electrolyte formulations on the market, the optimal strategy for electrolyte reuse involves selectively extracting individual components from the electrolyte mixture. This selective extraction represents a potential area for future research in the field of electrolyte recycling, with cost analysis being conducted in parallel.
2.2. Nickel, Manganese, and Cobalt Salts for Li-Ion Cathodes
Nickel, manganese, and cobalt are constituent elements of many cathode chemistries for rechargeable batteries. All of these relevant elements must ultimately be extracted from the earth via the mining of ore. The concentration and quality of ore are determined by geological history, as deposits are unevenly distributed around the world in varying degrees of quality and concentration. At the outset, the two most important chemical characteristics of any ore are (1) the concentration of the relevant element in the ore and (2) the presence of unwanted impurities in the ore. Besides concentration, the financial and environmental costs for refining the ore are direct functions of the quantity and type of impurities present in the ore. Some impurities may be in high concentration but relatively easy to remove, while other impurities may be in low concentration but difficult or even impossible to remove. Impurities can include but are not limited to cations such as sodium, potassium, or calcium or heavy metals such as molybdenum or chromium, just to name a few.
In the example of manganese processing, the concentration of the raw ore is critical. Because the raw manganese ore must be roasted to make the manganese soluble in acid for leaching, the amount of energy required to synthesize a specific quantity of battery-grade manganese is ultimately a direct function of the concentration of manganese in the raw ore. Low-concentration ore still requires the same amount of roasting on a per-weight basis as high-concentration ore while producing significantly less manganese via leaching. The problem of low-concentration ore bedevils the processing of nickel, cobalt, and other materials. At a sufficiently low concentration, the processing of the ore becomes economically unviable.
For manganese processing, the concentration of calcium in the ore is problematic. Because manganese is extracted via a leaching process, high quantities of calcium will lead to significant blockages in the plant’s pipes and equipment, costing time, energy, and money to clean and maintain them. Other impurities are added as part of the extractive or refining process. For example, in manganese processing, manganese sulfate (MnSO4) is created as an intermediate product. If the process is not carefully controlled, then the final product will contain sulfate in varying concentrations.
Nickel is more expensive than manganese and still dominates high-energy Ni-rich cathode materials. Only high-content Nickel I is used by battery manufacturers to make nickel sulfate (NiSO4), a precursor for NMC811 synthesis. Nickel II, with lower nickel content, is mainly utilized in stainless-steel production. Innovations to convert Nickel II, such as nickel pig iron, to nickel matte and eventually NiSO4 for cathode manufacturing with cost-efficient approaches will be of great importance from the point of view of relieving the challenge of the battery-grade nickel shortage.
Cobalt is still used, although its use is continuously decreasing, in cathode materials. Two-thirds of the global Co supply is mined in Africa, where there are concerns about child labor and harm to workers’ health. Recycle and reuse of those valuable metals in spent car batteries will help. But we also need to understand that, for now, mining those metals is still less expensive than recycling them from used EV battery packs. So the key is to develop cheaper ways to recycle LIBs and make them competitive with freshly mined ones. In addition, scale will help reduce the recycling cost, especially if environmental concerns can also be resolved.
It is not well understood whether some of the common impurities in nickel, cobalt, and manganese can be used as dopants, which should be determined by the type and concentrations of those impurity “dopants”. Ores from different geographical locations may contain various contents of the same element impurity, so fast analysis and quantification of impurities is needed.
2.3. Iron and Phosphorus Materials as Precursors for Polyanion-Based Cathodes
Olivine cathodes, especially LiFePO4 (LFP), have been widely commercialized for EVs in Asian countries and have been deployed for grid applications. The majority of industrial methods for the production of LiFePO4 utilize solid-state processes, which require the selection of appropriate raw materials of iron, lithium, and phosphorus sources for calcination. Based on the iron source, the process can be categorized into iron phosphate (FePO4), ferrous oxalate (FeC2O4), and ferric oxide (Fe2O3) routes.
For the preparation of iron sources, iron ore, such as hematite (Fe2O3) and magnetite (Fe3O4), is typically used as a raw material to extract elemental iron. Compared to other non-ferrous metals such as Ni and Co, the metallurgy of iron is more large-scale and the resources are more abundant. In the subsequent manufacturing processes, the metallic iron reacts with different acids to form iron salts, such as FeCl2, FeSO4, and so on. The ferrous oxalate-based sintering process is currently the most widely used industrial route due to its simplicity and scalability. The preparation of ferrous oxalate can be achieved by adding oxalic acid to an iron salt solution.
Other precursors for LFP production include lithium carbonate (Li2CO3), lithium hydroxide (LiOH), and lithium acetate (CH3COOLi), which are commonly used as lithium sources. Ammonium phosphates serve as phosphorus sources, including ammonium dihydrogen phosphate (NH4H2PO4) and diammonium hydrogen phosphate ((NH4)2HPO4). H3PO4 is also directly used as a phosphorus source for LFP synthesis, but impurities easily dissolve in H3PO4, so strict quality control is required. The production of ammonium phosphates is usually driven by the neutralization reaction of phosphoric acid (H3PO4) with ammonia in the reaction tower. Phosphoric acid is a critical industrial raw material for the production of phosphorus sources for polyanion-based cathodes, which can be produced by treating phosphate rock (commonly apatite) with sulfuric acid.
For the route in which iron phosphate is chosen as iron source, FePO4 precursor is first synthesized by the reaction of ferrous sulfate with purified phosphoric acid or phosphates, e.g., NH4H2PO4. Then the iron phosphate precursor is subsequently calcinated with lithium salt such as Li2CO3, carbon black, and/or organic additives (glucose, sucrose, or starch) to coat carbon on as-produced LFP to enhance its electronic conductivity. For the processes that use ferric oxide as the iron source, lithium dihydrogen phosphate (LiH2PO4) is used as both the lithium and phosphorus source. Ferric oxide is manufactured from the purification of hematite ore materials, for which purity is critical. The production of lithium dihydrogen phosphate also involves phosphoric acid (H3PO4) as the reactant, which reacts with lithium hydroxide (LiOH) to form LiH2PO4. ,,
Compared to LiFePO4, LiMnPO4 offers a higher operating voltage (∼4.1 V), which increases the theoretical energy density (21% higher than LiFePO4), but it is limited by poorer ionic and electronic conductivity compared to its LiFePO4 countpart. LiMn x Fe1–x PO4 combines the characteristics of both LiFePO4 and LiMnPO4 by the substitution of Mn2+ at the transition metal Fe2+ site, providing a balance between energy density and cycling stability. The synthesis of lithium manganese phosphate (LiMnPO4) and lithium manganese iron phosphate (LiMn x Fe1–x PO4) can also be industrialized with the further introduction of manganese sources using solid-state methods. Common manganese sources include Mn(H2PO4)2, MnCO3, and Mn(CH3COO)2. Manganese ores, such as pyrolusite, rhodochrosite, and manganite, can served as the primary raw materials for obtaining these compounds, which has been discussed in an earlier session. Iron sources still include iron phosphate, ferrous oxalate, and ferric oxide, with corresponding manufacturing routes. ,
A key advantage of olivine cathodes is the abundance of precursor materials needed to manufacture these cathodes. The precursor raw materials are better distributed globally than Ni and Co raw materials. A shortage of phosphorus for battery applications is unlikely to happen on a global scale. However, supply chain security may call for more distributed phosphate processing facilities. Meanwhile, the supply of phosphate for battery manufacturing should not impact fertilizer production, which is the primary consumer of phosphorus. Fe and Mn are both abundant and well distributed globally. Fe/Mn precursors used to produce olivine cathodes can come in different chemical forms, including metal, metal phosphates, metal sulfates, and metal oxides. The wide selection space of Mn and Fe precursors opens possibilities for manufacturing innovations. Different manufacturing technologies could use different precursors, allowing for a new process design that could reduce manufacturing cost, liquid and gaseous waste, and energy consumption.
2.4. Artificial and Natural Graphite
Natural and synthetic graphite are indispensable materials in the construction of LIBs, albeit with distinct characteristics and production methodologies. Usually, many energy-intensive process steps are involved in the production of graphite (Figure ), which is also carbon intensive.
3.
Flowchart illustrating the manufacturing process for graphite anode materials.
Natural graphite, sourced from naturally occurring deposits, primarily consists of carbon and is extracted through mining operations worldwide. Conversely, synthetic graphite is meticulously produced via the carbonization of organic precursors such as petroleum coke or coal tar pitch. , This process yields graphite with highly controlled atomic structures and purities, ensuring consistency in its electrochemical properties. While natural graphite may exhibit varying compositions and structures due to its geological origins, synthetic graphite offers a more uniform structure and purity, enhancing its suitability for the most demanding battery applications. Despite its typically lower cost stemming from mining processes, natural graphite often suffers from higher impurities and inconsistent performance, whereas synthetic graphite’s engineered properties deliver superior electrochemical performancemost notably longer cycle life, less swelling during charge/discharge cycles, and less gas generation and therefore less pressure buildup in the cell. The choice between natural and synthetic graphite hinges on factors such as performance requirements, cost considerations, and application-specific needs within the lithium-ion battery industry. Approaches that can efficiently remove the unwanted impurities and enhance the electrochemical properties of natural graphite without increasing the total cost will be attractive to industry processing.
2.5. Hard Carbons
In addition to natural and synthetic graphite, hard carbon has been extensively studied as an alternative material for LIB anodes. Hard carbon is a type of noncrystalline carbon material distinguished by its inability to graphitize or form ordered structures, even at temperatures exceeding 2800 °C. Hard carbon is typically derived from biomass or petroleum residues through controlled pyrolysis processes, resulting in a disordered carbon structure with a high capacity for lithium storage.
The interlayer spacing and porosity of hard carbon and, therefore, its capacity can be adjusted by changing the processing temperature, which enables customization based on the desired application. The structure, being more amorphous than that of graphite, can provide advantages with respect to rate performance and capacity. However, challenges such as lower initial Coulombic efficiency and limited understanding of its structural evolution during cycling, leading to faster degradation, warrant further research and optimization efforts to fully exploit the potential of hard carbon in LIBs. The exploration of hard carbon alongside natural and synthetic graphite represents a significant avenue for advancing battery technology and addressing the growing demand for energy storage solutions.
Furthermore, the investigation into natural graphite, synthetic graphite, and hard carbon transcends LIBs, encompassing sodium-ion battery systems. Despite LIBs dominating the energy storage landscape, sodium-ion batteries have garnered increasing attention due to the abundance and cost-effectiveness of sodium resources that diversify the supply chain of battery technologies. However, although sodium is cheaper and more abundant than lithium, the necessity of utilizing hard carbon as the anode material in Na-ion batteries sacrifices the cost reduction brought by using sodium because hard carbon is much more expensive than graphite, which is a common anode in LIBs. More basic research is necessary to reduce the manufacturing cost of hard carbon for Na-ion batteries. Recent studies − also show promising results from graphite when used in optimized Na-ion batteries. If graphite can be directly utilized as the anode for Na-ion at large scale, the cost of Na-ion battery systems will be considerably reduced.
The fundamental operating principle of sodium-ion batteries mirrors that of lithium-ion batteries, with sodium ions shuttling between the cathode and anode via the electrolyte during charge and discharge cycles. Analogous to their lithium counterparts, sodium-ion batteries necessitate suitable anode materials for effective energy storage. However, in sodium-ion batteries, the movement of sodium ions within the stack of graphene sheets constituting graphite is impeded.
While a few different cathode materials can be utilized for sodium-ion batteries, hard carbon is currently the only viable anode active material to realize the commercial viability of sodium-ion batteries. Due to its large spacing between the graphene sheets compared to graphitic carbon, hard carbon possesses a higher sodium-ion storage capacity, reaching up to 300 mAh/g. The storage mechanism of Na+ in hard carbon remains a subject of ongoing debate, with several models proposed, including the “intercalation–filling”, “adsorption–intercalation”, “adsorption–filling”, and “three-stage” models. These models suggest various ways in which Na+ ions interact with hard carbon electrodes during charge and discharge cyclessome propose intercalation into graphitic layers during specific phases, while others suggest adsorption at surface or defect sites. Each model provides valuable insights into the complex behavior of Na+ storage in hard carbon electrodes, underscoring the need for further research in this area. −
The carbonization process plays a key role in tuning the microstructures of hard carbon anode materials. The heat treatment usually happens from a few hundred Celsius degrees to about 2000 °C. For comparison, the temperature used to prepare competitive synthetic graphite is usually greater than 3000 °C, , half the temperature of the surface of the sun. Specialized furnaces are needed to tune precisely the temperatures and heating environments to precisely control the electrochemical properties of carbon. The temperature needed for hard carbon production also depends on the precursors, which contain various impurities. , Sucrose, glucose, polymer resin, and natural biomass have all been used to synthesize hard carbon. From the manufacturing cost aspect, it may be worthy of study to find the lowest temperature needed for each different precursor to produce hard carbons with balanced performance. Alternatively, a low-temperature hydrogen reduction method or Joule heating to shorten the heating time also may help to further reduce the manufacturing cost by saving energy.
While hard carbons derived from biomass provide opportunities for cost reduction, the attainable purity, tap density, and surface area vary depending on the different biomass precursors. A stable massive supply of biomass raw materials with consistent quality and controlled impurities remains a challenge for hard carbon manufacturing. Nevertheless, the abundance of carbonaceous materials and deepening understanding of the nanostructures available ensure that the technology will continue to advance as new precursors and processing technologies are developed to continue to provide a high-energy, long-lasting, environmentally friendly, and sustainable anode material for years to come.
2.6. Silicon-Based Anodes
Silicon-based anodes are at the forefront of research and development for next-generation LIBs with a targeted cell level energy density of about 350 Wh/kg. However, the practical implementation of silicon anodes is hindered by challenges such as volume expansion, structural disintegration, , and the instability of the SEI. , Novel synthesis and manufacturing technologies have been developed and can address these issues to a certain extent. There are a few key production pathways for Si-based anodes, involving silane gas deposition, silicon oxide reduction, and recycling silicon from solar panels.
Silane (SiH4) is an important gas-phase precursor in producing high-purity silicon for battery anode applications. The global production of silane is dominated by a few major chemical companies, such as Air Liquide, Dow Chemical, REC Silicon, and Wacker Chemie, through the operation of large-scale facilities capable of producing high-purity silane gas. Key production facilities are in regions with a strong presence in the semiconductor and photovoltaic industries, including the United States, Europe, and East Asia. The process of using silane to create a silicon anode often involves chemical vapor deposition (CVD) to deposit silicon onto a substrate. This method is advantageous due to its ability to produce uniform, high-purity silicon coatings with controlled texture. Applying this method to a porous carbon substrate has also been demonstrated to be effective for manufacturing C/Si composites with controlled composition and morphology. The production and supply of silane gas, however, can be limiting factors. Specifically, silane’s properties as a highly flammable, reactive, and toxic gas make it essential to handle it with extreme caution. Co-locating a Si anode manufacturing facility with a silane plant(s) is probably the most practical approach to address these challenges, but it adds obvious constraints.
While silicon, at about 28%, is the second most abundant element in the Earth’s crust, it is mainly found in the silica (SiO2) or silicate (SiO4) forms. The use of silicon oxides as precursors for manufacturing silicon-based anode materials has gained significant attention. SiO x (silicon suboxide, x < 2) anodes represent a hybrid approach, balancing the high capacity of silicon with the stability of oxides. The reduction process can be done through various chemical and thermal processes, incorporating carbon to improve conductivity and buffer volumetric changes. It often results in a composite material, where silicon is dispersed within an oxide matrix. This structural hierarchy can be carefully tailored to optimum structural robustness. Despite improved cycle stability and moderate capacity, SiO x anodes typically have lower initial Coulombic efficiency and complex synthesis requirements which complicate manufacturing process and cost.
Recycling silicon from end-of-life solar panels is an interesting approach to produce silicon-based anodes, addressing both the supply chain sustainability and environmental impact of silicon production. As of 2024, many of the first-generation solar panels installed in the early 2000s are reaching the end of their typical 25-to-30-year lifespan. This initial wave of decommissioning creates a substantial volume of retired solar cells. The recycling process involves collecting and mechanically processing silicon wafers from decommissioned solar panels, followed by chemical and thermal treatments to remove impurities. The purified silicon is then processed into fine particles or composites, enhancing its electrochemical performance and stability. Recycling silicon from solar panels reduces waste and the need for raw material extraction, contributing to a circular economy. However, the variability in the quality and composition of silicon from solar panels necessitates rigorous purification and standardization processes. Overcoming challenges related to cost, safety, and processing complexity is crucial for the commercial viability of these technologies.
2.7. Lithium Metal Processing
Lithium metal has become an important anode for next-generation rechargeable lithium metal batteries that potentially can double the cell-level energy of state-of-the-art LIBs. The foil format of Li metal is processed by extrusion or a vapor-based technique. Before that, lithium ingots are obtained from the electrowinning process in a molten salt electrochemical cell. , Because of the different processing technologies, surface roughness, impurities, the type and amount of metal alloys, and the corresponding tensile stress of lithium metal foils vary.
Metallic lithium is typically produced through the electrolysis of lithium chloride (LiCl), which is derived from Li-containing minerals or lithium-rich brines. The electrowinning process takes place in a LiCl–KCl eutectic molten salt (400–450 °C), with a graphite anode and stainless-steel cathode (Figure a). ,, The lighter lithium metal floats to the surface of the molten salt and is collected, while chlorine gas is processed or vented on the anode. After being collected and cooled, the lithium ingot will be stored in an inert environment to prevent oxidation before further processing.
4.
(a) Illustration of molten salt electrolysis to produce Li metal ingots. Reproduced with permission from ref . Copyright 2020 Nature Publishing Group. (b) Different processing methods to produce lithium metal foils from lithium ingots or similar. Reproduced with permission from ref . Copyright 2023 Nature Publishing Group.
In Li metal battery applications, the methods for processing Li foil are crucial for both performance and cost. Currently the widely applied methods can be divided into mechanical processing, evaporation/sputtering deposition (Figure b), ,, and electrochemical plating. The calendaring process can reduce the Li foil thickness effectively from about 500 to 50 μm. Further reducing the thickness of the Li foil to below 50 μm will need different approaches such as sputtering, physical vapor deposition, and thermal evaporation. However, challenges exist in reducing the deposition time and cost while producing lithium foil on a large scale, all of which are rooted in lithium’s low melting point of 181 °C and high vapor pressure at low temperatures (10–4 Torr at 407 °C). Besides these three methods, Livent’s LIOVIX or SLMP offers a flowable, stabilized lithium composition mixed with binders and solvents. This enables the direct printing of lithium layers onto current collectors with precise, controlled loading. Another recently reported method, tape casting of molten lithium, involves spreading molten lithium onto a substrate using a doctor blade system, forming thin films (5–50 μm) as the metal cools. Under a processing temperature of 275 °C, the formation of the lithiophilic Li alloys layer enables the self-wettability toward the molten Li, allowing for the fabrication of ultrathin Li films.
2.8. Sulfur and Sulfides for Next-Generation Lithium Batteries
Sulfur materials, in the form of elemental S8, small-molecule S x (x < 4), or sulfurized polyacrylonitrile (SPAN), are promising candidates for transition-metal-free cathodes. − When coupled with a lithium metal anode to form a Li-S battery, this technology can theoretically deliver a gravimetric energy density of 2500 Wh kg–1 at the material level, with demonstrated practical cell-level energy exceeding 500 Wh kg–1. Additionally, sulfur is an earth-abundant element and is readily available as a massive byproduct of the petroleum/natural gas industries. Another advantage of sulfur compared to transition-metal-oxide cathodes is its ease of recycling via direct sublimation, which is both energy efficient and cost efficient.
Despite these advantages, manufacturing Li-S batteries that meet performance metrics for practical use still faces significant obstacles, including limited cycle life, low rate-capability, severe self-discharge, and potential safety concerns. A significant gap still exists between materials discovery and cell performance improvement. Nanosized and highly porous host materials have been widely adopted to enhance sulfur utilization in sulfur cathodes. Some of the host materials are very expensive, sacrificing the cost advantages of sulfur itself. More importantly, those highly porous hosts bring additional practical challenges for manufacturing high areal capacity electrodes (>5 mAh cm–2), as they need a large excess of inactive binders to “glue” the nanoparticles. A detailed cost analysis of Li-S battery technology including the processing cost will be very helpful.
All-solid-state batteries (ASSLBs) employing high-energy cathodes and metallic Li anodes have a good potential to enable high-energy Li metal batteries with enhanced safety attributes. Some inorganic solid-state electrolytes (SSEs) possess high Li+ transference number (∼1.0), low activation energy (<0.3 eV), and conductivities even higher than those of conventional organic liquid electrolytes. Although oxides are intrinsically more stable than sulfides, sulfide-based SSEs (S-SSEs) are arguably more viable for bulk-type ASSLBs. This stems from sulfides’ low material density, low elastic modulus, and high ionic conductivity, which allow its intimate contact with active materials and practical processability through slurry or dry processing. , Following the work on Li10GeP2S12 (LGPS), a series of sulfide-based compounds displaying extremely high ionic conductivities (>10–2 S/cm) have been developed. , By using surface-coated cathodes, stable cycling of LiCoO2 cells, high-power NMC cells, and high-energy NMC cells have been demonstrated in pouch cells, proving the viability of S-SSEs for high-performance ASSLBs. S-SSEs are also compatible with sulfur both chemically and electrochemically, making them feasible for S cathode fabrication without additional cathode coating. ,
Despite the advancement in SSEs, significant manufacturing challenges, including materials’ mositure sensitivity, Li/SSE interfacial stability, and scalable procesing of SSE films and electrodes, need to be addressed before the large-scale deployment of SSEs. , To reach a cell level energy of 500 Wh kg–1 in a 2 Ah pouch cell, the areal capacity of S cathodes needs to be greater than 8 mAh cm–2. Although S-SSEs allow Li metal cycling at certain current densities and areal capacities, stable Li cycling at capacities matching those of high-areal-capacity S cathodes is still beyond reach. This is why long cycling of high-areal-capacity cathodes usually requires the use of indium as the interface for Li metal anodes to mitigate the interfacial problems, which unfortunately sacrifices cell energy due to the heavy material (In: 7.3 g/cm3) and its high working voltage (0.6 V vs Li). Comprehensive strategies built on innovations in material development, interfacial engineering, chemo-mechanical management, and applicable processing are essential to overcome the barriers with Li faced by manufacturing practical ASSLBs.
Another significant challenge in manufacturing ASSLBs is developing feasible processing technologies for the scalable and efficient fabrication of ultrathin SSE separators, high-energy electrodes, and full cells. , Currently, most ASSBs are evaluated at lab scale using binder-free electrolyte pellets, which are very thick (>100 μm) to ensure appropriate mechanical strength. This method is impractical due to poor processability and a significant sacrifice in cell level energy, in addition to the cost concern. Employing innovative manufacturing techniques to fabricate thinner, yet robust, SSE membranes will help address this challenge. The wet slurry coating process, a well-established scalable approach in LIB manufacturing, requires a small amount of polymeric binder and sufficient solvents to form a uniform slurry. However, a chemically compatible solvent and polymeric binder are necessary to minimize the side effects from processing SSEs. Considering sulfides’ moisture sensitivity and chemical instability, dry processing is usually preferred. This method has been adopted in commercial supercapacitors but is not widely used in the fabrication of ASSBs, particularly with S-SSEs. Without solvent, homogenizing the distribution of SSE powders and polymeric binder particles is very challenging when aiming to achieve a high-quality freestanding film with a very lean amount of binder (<1 wt%). Through fibrillation of polytetrafluoroethylene (PTFE), sufficient binding for high-mass-loading electrode fabrication can be achieved with a very small amount of binder. However, the PTFE fibers in the processed SSE separator easily react with Li metal chemically, propagate through the separator, and short the cell. Therefore, compatible binder materials, feasible processing technologies, and dedicated equipment are required to enable the scalable manufacturing of ASSLBs.
2.9. Copper and Aluminum Processing: From Electrowinning to Recycling
Current collectors are pathways for conducting electrons but are inactive components, which means their weight needs to be reduced as much as possible without sacrificing their mechanical and electric properties in order to improve cell-level energy. Today’s lithium batteries use copper and aluminum as the current collectors for anodes and cathode, respectivelysodium batteries can use aluminum for both electrodes because aluminum does not form an alloy with sodium at the same electrochemical potentials driving Na+ ions to go into or come out of hard carbon structures. Because copper (density of 8.94 g cm–3) is much heavier than aluminum (density of 2.7 g cm–3), decreasing the thickness/weight of copper current collectors is very effective for improving cell energy. In addition, copper is more expensive than aluminum; thus, the following discussion will focus on copper mining and processing.
Mining of copper is complicated because the ore usually contains less than 1% of copper. Depending on the types of ore, copper oxides or copper sulfides, different extraction methods are utilized to yield 99.99% pure copper. Copper oxides are more abundant in the ore, but they have a lower concentration of copper. Copper sulfide ores contain more copper but are less abundant. Copper processing operations consume a huge amount of water and are very energy-intensive in the mining industry.
A hydrometallurgical leaching process is usually used to extract copper from copper oxide ores. Water-based solutions are used to extract and purify copper from oxide ores at ordinary temperatures. As shown in Figure , the process goes through three steps: heap leaching, solvent extraction, and electrowinning. Heap leaching is generally used for low-grade ores like copper oxide by utilizing percolating chemicals such as sulfuric acid to leach out metals. Sulfuric acid is sprayed through sprinklers on top of a heap pile built by crushed ores on a slight slope. The acid diffuses through the heap and dissolves the copper from the ore. The resulting solution consists of sulfuric acid and copper sulfate. The copper in the leaching solution is further concentrated by solvent extraction. Two immiscible liquids (leaching solution and another solvent) are mixed vigorously and allowed to separate, with different minerals left in each liquid. The copper sulfate moves from one liquid to the other solvent due to different solubilities, leaving impurities in the original leaching solution, which will be further recycled to extract more copper out. Electrowinning is then applied to electrochemically plate copper metal out of copper sulfate solutions. An inert electrode is usually used as the counter electrode in the electrowinning process. Common materials include lead alloy and titanium plates coated with RuO2, IrO2, or PbO2. Oxygen is generated on the inert electrode side during the electrowinning process. , The presence of iron impurities such as Fe2+ will be oxidized on the anode side, forming Fe3+, which then goes back to the cathode and gets reduced back to Fe2+, forming a shuttle and thus lowering the efficiency of cooper plating. Pb/Sb alloy is therefore used to retard the reoxidation process from Fe2+ to Fe3+. ,
5.
Manufacturing processes to convert different copper ores into pure copper.
Sulfide ores are usually processed using the pyrometallurgy method. Heat and intense energy are used to extract and purify copper from the sulfide ores. Four steps are involved, as presented in Figure : froth flotation, thickening, smelting, and electrolysis. Sulfide ores are processed repeatedly into fine sands before liquid is added to form slurries. Copper minerals are separated from rocks through a froth flotation process. Chemical reagents are then added to the slurry, which bind to the copper particles, making them hydrophobic. Copper-rich particles, created by blowing air into the slurry, rise to the top. The froth is skimmed off for thickening, which allows the solids from bubbles to sink to the bottom of a large tank called a thickener. After filtering, the concentrated copper is ready for smelting at up to 2300 °F, leading to the formation of molten liquids. The heated liquid goes through a slag-settling process. This step separates the matte and slag from the molten liquids. The matte is a mixture of copper, sulfur, and iron, while the slag is a dense and glassy material composed of iron, silica, and other impurities. About 0.3–6% copper is also trapped in the slag. The flotation method is usually applied to recover metallic copper resources from the copper slag in industrial use. Iron and sulfur in the molten matte then burn off, concentrating the copper content to 98%. After further smelting to burn off oxygen, the molten copper now has 99% purity and is next refined through electrorefining to electrochemically dissolve copper from a less pure copper anode (99% copper purity) and redeposit it on the cathode side, which has 99.99% purity of copper.
When copper or aluminum foils are too thin, it becomes challenging to handle and coat active materials, because of mechanical issues. So, the challenge is to manufacture ultrathin metal foil (a few microns) that is still sufficiently durable for battery use. The tabbing areas of those ultrathin current collectors also raise challenges of increased resistance and heat generation. Copper foils used for Li batteries are often manufactured by rolling or electrodeposition. Rolled Cu foils provide a double-sided smooth surface but may be too expensive for batteries. Electroplated Cu is much cheaper, and more importantly, it has higher surface roughness, which benefits electrode coating. Besides making copper/aluminum foil thinner and thinner, other approaches include metal mesh current collectors, perforated current collectors, and composite current collectors. An additional advantage of perforated current collectors is that spikes are left on both sides of the perforated metal foils to help integrate electrodes on the two sides together. The integrated structures enabled by perforated metal current collectors also provide the benefit of extending the electron conduction “highway” inside the entire electrode. Composite current collectors are prepared by coating super thin Cu layers (a few hundreds of nm) on both sides of a thin polymer film, e.g., polyimide. As the current collectors become thinner and thinner, the tabbing area needs appropriate design not only for proper welding of thin tabs together but also for managing heat due to resistance increases of each thin tab.
The production of aluminum and copper metals is highly carbon-intensive: about 12 tonnes of CO2 equivalents (CO2e) per tonne of aluminum and 6 tonnes of CO2e per tonne of copper. A more sustainable way to obtain such metals is urgently needed. Today’s aluminum production involves high-temperature electrolysis, also known as the Hall–Héroult process, which consumes a significant amount of electricity (over 14 MWh per tonne of aluminum)using renewable electricity can reduce a large portion of CO2 emission. The high-temperature electrolysis process itself is very emission-intensive because the carbon anode used in the electrolysis cells reacts with oxygen and electrolytes to form CO2 and perfluorocarbons which have global warming potential (GWP) a few thousand times as strong as CO2the amount of CO2e released annually during the high-temperature electrolysis process for global aluminum production is more than the total combined annual CO2e release from all the four states in the U.S. Pacific Northwest region (WA, OR, ID, MT). Research to replace consumable carbon anodes with inert oxygen evolution electrodes is ongoing, but significant effort is still needed to move the technology into the market.
Aluminum and copper can be recycled repeatedly without losing performance; they have an infinite recyclable life, and their common recycling is much less carbon intensive. Recycling copper and aluminum from batteries is still at an early stage before commercialization but is attracting significant attention. Copper and aluminum recycled from other sources are often coated with insulation or mixed-metal alloy scraps. For battery current collector applications, upcycling of these mixed-metal alloy scraps to pure metal is needed but challenging. For example, only two industrialized technologies in use are capable of refining aluminum: the Hoopes process and the segregation method, which are limited by either purity, long upcycling duration, or waste of aluminum recourse. New processes capable of upcycling aluminum and copper for battery current collector applications are needed.
3. Science and Engineering in Battery Materials Manufacturing
3.1. Cost-Oriented Fundamental Research
Not only is understanding cost components important for battery manufacturing, but it also provides new insights for scientists and engineers to identify the key directions to support industry in lowering production costs. In general, materials costs account for 60–80% of the total cost, while manufacturing costs are about 20–40% that of conventional LIBs. To reduce the manufacturing cost of batteries, both materials and processing need to be cost efficient. A completely different mindset is necessary to revisit science and engineering solutions behind the pressing challenges that industry faces, which are all related to cost reduction.
3.1.1. Processing Lithium Metal for Rechargeable Lithium Metal Batteries
Lithium metal has been used in primary batteries such as lithium carbon fluoride (Li/CF x ) and lithium thionyl chloride (Li/SOCl2) batteries, which have been commercialized for years. For example, Li/CF x batteries have high energy density and long storage life, making them good for military, sensor, and space applications. For Li/SOCl2 batteries, the electrolyte is based on sulfonated thionyl, which also serves as the cathode. Li/SOCl2 batteries work at both very low and high temperatures with excellent durability, making their usage broad, especially for industrial applications and defense.
In recent years, rechargeable lithium metal batteries have also received much attention. Good progress has been demonstrated in both energy density and cycling stability. The safety of rechargeable lithium metal batteries has not been fully investigated yet, but one of the major root causes of lithium metal battery safety is the pulverization of lithium metal after extensive cycling, which also impacts the cycle stability. This means that if lithium pulverization can be mitigated or eventually eliminated in rechargeable lithium metal batteries, safety and cycling will both be improved. Although challenging, it is worthy of more effort to fully understand the stripping and electroplating process through dedicated fundamental research which needs to be conducted at testing conditions relevant to practical cells.
In lithium metal batteries, the lithium anode mainly exists in the form of a foil. Lithium metal foils are derived from lithium ingots through either an extrusion process or vapor-based deposition. Depending on the technology used, lithium foils with different thicknesses can be processed either for prelithiation of the silicon anode or as a lithium metal anode directly. As discussed earlier, lithium metal thicker than 50 μm is relatively convenient to produce and has been used in rechargeable lithium metal batteries. Lithium metal thinner than 50 μm is more challenging to manufacture, considering thickness homogeneity at an enlarged scale, control of defective sites, and surface roughness. Innovations are needed to develop cost-efficient processing technologies to produce thin lithium metal foils.
For free-standing lithium foils, a special substrate is needed to support sticky lithium metal which needs to be detached conveniently to release it. For lithium foil attached to copper current collectors, a protective film is still needed to cover the surface of the lithium metal. If the protective film can directly serve as the separator or even the solid-state membrane to be used in solid-state batteries, the add-on value brought by this functional protective layer will be nontrivial compared to the use of pure lithium metal foil itself. Lithium metal alloys have been investigated and compared with pure lithium metal in terms of their reversibility and dendrite formation. Because the solid-state electrolyte interphase layers still form and are being accumulated within the alloys, the cycling improvement by using different alloys is limited, if any. A different look at lithium metal alloys from the tensile stress and surface stickiness points of view may bring some new insights toward reducing the manufacturing cost of thin lithium metal or alloy foils.
Lithium ingots are derived from a lithium salt such as lithium chloride (LiCl) through molten salt electrolysis (Figure a), which is still the only commercialized production approach for lithium metal. LiCl is usually converted from Li2CO3 by using a chlorinating agent such as hydrogen chloride. Thus, lithium metal is intrinsically extracted from hard rocks or brines, which are the sources for producing Li2CO3, as we discussed earlier. The electrolysis process to produce lithium metal is quite straightforward. The cathode used is a steel on which liquid lithium will be electroplated (Figure a). A graphite electrode is employed as the anode on which the chlorine gas evolves. The overall electrochemical reaction is shown in Figure a. The electrolyte used in the electrolysis is a mixture of LiCl and KCl. The former participates in the electrochemical reactions on both electrodes, while the latter is a supporting electrolyte. One can imagine that this process is energy and capital intensive. The generation of chlorine gas also poses environmental concerns. A concept of carbonate-to-metal lithium has been proposed to convert lithium directly from lithium carbonate, thus removing the harmful chlorine gas in the process. Directly reprocessing lithium metal scraps into ingots has seen some success, although it may still need some time to understand whether lithium scraps from different suppliers will produce the consistent quality and purity needed for battery applications. The manufacturing cost of those new innovative technologies needs to be compared to that of the state-of-the-art electrowinning process.
3.1.2. Low-Cost Processing of Phosphate Cathode (LiFePO4/LiFe x Mn y PO4/LiMnPO4)
Solid-state synthesis of LiFePO4, LiMnPO4, or their mixed solutions is still the mainstream approach. However, the solid-state method usually lacks good control over particle morphology, especially at the primary particle scale. Therefore, the solid-state method, when used in practical production, usually requires repeated mechanical grinding and high-temperature processing. These tedious processes improve the final product quality but also increase energy consumption during production. There is ample room for new innovations in solid-state manufacturing. Technologies that can decrease the manufacturing temperature and duration will further decrease the cost of these materials and improve the environmental friendliness of the production. Solution-based methods, such as sol–gel, coprecipitation, and hydro-/solvothermal techniques, may seem appealing, as they could offer better morphological control. However, liquid waste management could be a challenge and often does not meet the “green chemistry” principles.
Further, during LFP synthesis, many impurities readily form, such as Li3PO4 and Li4P2O. FeP, Fe2P, and Fe3P are also found as impurities which develop under the reducing environment and high temperatures. In addition, oxygen leakage during high-temperature synthesis also leads to the formation of Fe2O3, while Li deficiency promotes the impurity of Li3Fe2(PO4)3. The roles of some impurities are still arguable. For example, Fe2P is reported to improve the electronic conductivity, while others believe that Fe2P formation needs to be suppressed as an impurity in the LFP product. Similarly, Li3PO4 and Li4P2O7 are also claimed to improve the rate capabilities of LFP, although they will impact the stoichiometry and thus usable capacity of LFP. These inconsistent observations highlight the importance of reassessing cathode materials in a battery system by designing specific full-cell protocols to ensure the electrochemical performance of the entire electrochemical cell is dominantly controlled by the cathode and cathode–electrolyte interphase (CEI) instead of the SEI or other limiting steps. Innovations are needed to realize impurity control of LFP with reproducible performance without increasing the manufacturing cost.
The chemical space of olivine cathodes has been well established. The design principles for olivine cathode active materials and electrode formulations have been well developed. Olivine cathodes are compatible with different cell formats, including prismatic, blade, and cylindrical battery types. BYD’s balde battery also provides a new direction for enhancing the utilization of cell space through structural design at the module level to increase the energy density of LFP-based chemistry. Due to the competition and significant lithium salts dropping, the cost of olivine cathode-based batteries has approached a new low in the past year, falling below $100/kWh in 2023. Although the prediction for the market share of different cathode materials could fluctuate, the use of olivine cathodes is likely going to increase, especially when high energy density is not a priority, such as electric buses and commuter vehicles. Reports have suggested that the overall carbon footprint of olivine-based batteries is lower than that of NMC-based ones. Further advancements in low-carbon manufacturing technologies, coupled with the adoption of renewable energy in production regions, could amplify the environmental benefits of these batteries. In the next decade, olivine-based lithium-ion batteries are likely to face some competition from sodium-ion batteries, although it is not clear which chemistry is safer due to the lack of “apples-to-apples” comparisons for safety testing. Continued innovation in olivine technologies from materials to electrode and cell levels remains crucial for maintaining their competitive edge.
3.1.3. NMC Synthesis and Scaleup
The dominant approach for NMC synthesis is the coprecipitation method, during which transition metal cations such as Ni2+, Mn2+, and Co2+ in their sulfate solutions are precipitated out into transition metal hydroxide precursors, followed by multiple steps of heating, washing, and annealing to obtain the final products. The coprecipitation method has been successfully adopted by industry in producing tons of NMC cathodes such as LiNi1/3Mn1/3Co1/3O2 (NMC111), LiNi0.4Mn0.4Co0.2O2 (NMC442), LiNi0.5Mn0.3Co0.2O2 (NMC532), and LiNi0.6Mn0.4Co0.4O2 (NMC622).
Although the coprecipitation method is a mature manufacturing process for NMC synthesis, there are still spots for scientists and engineers to work on to further reduce the production cost and impact on the environment. For example, a huge amount of wastewater is generated during the coprecipitation step. Although the wastewater can be recycled and reused, the ideal situation would be to minimize or eventually eliminate wastewater generation. In addition, LiOH·H2O and LiOH are usually preferred as the salt precursors due to their low melting points compared to Li2CO3, especially for high-nickel NMC synthesis. However, a huge amount of corrosive water is generated during large-scale production, which becomes a key challenge in cathode manufacturing.
In addition to conventional polycrystal NMCs, single-crystal NMCs have been broadly investigated in the past few years. Especially for nickel-rich NMCs, challenges such as cracking, moisture sensitivity, and gassing plague their large-scale use in batteries, but all originate from phase boundaries. Single crystals without any grain boundaries are believed to at least mitigate those problems associated with nickel-rich NMCs. However, single-crystal synthesis is more expensive than that of polycrystals, considering the reaction media and calcination time at high temperatures needed for single-crystal growth. A recent work utilizing Li2O instead of conventional LiOH as the salt precursor provides a new direction for single-crystal NMC synthesis by taking advantage of an interesting sublimation phenomenon of Li2O. The self-generated Li2O vapor from sublimation quickly diffuses and comes in contact with the surfaces of all other precursor particles, which leads to the rapid sintering of single-crystal NMCs. More innovations are needed for lithium salts to address manufacturing challenges and eventually reduce the cost of NMC production.
For the reasons discussed above, all-dry synthesis of NMCs , is now being revisited with an intention to simplify the manufacturing process. Transition metal oxides are directly mixed with Li2CO3 followed by calcination. All-dry synthesis is not new and has been used for LiCoO2 production and high-voltage spinel. However, LiCoO2 has only one transition metal, Co, in the structure, while NMCs have three transition metals that need to be mixed homogeneously at the atomic level to ensure the structure stability and capacity from the mixed layered oxides, which is why coprecipitation is employed to prepare NMC precursors. In addition, all-dry synthesis may pose a challenge for impurity control due to contamination from milling media, and it can damage the material structure or its interface upon cycling. Nevertheless, if the milling energy and media can be well controlled, the direct solid-state synthesis will provide a new direction for scaling up NMCs; a detailed cost analysis is still necessary because transition metal oxides used in all-dry synthesis are also derived from sulfates by heating or other processes that introduce additional steps in the entire manufacturing process. Alternatively, NMC622, with medium Ni, content is also being revisited to enhance its stability at high voltages (>4.2 V vs graphite) to extract high energy similar to that of NMC811 but without all the problems that NMC811 currently has, which will need a deep understanding of both lattice structure stability and CEI properties at elevated voltages.
3.1.4. Impurity Control
As mentioned repeatedly in the above discussions, impurity detection and control are of great importance to ensure the consistently good quality of battery materials and components. Throughout the battery manufacturing process, from material synthesis to cell assembly, stringent detection, monitoring, and control of impurities are critical, as these contaminants significantly influence battery performance, safety, and lifespan. Depending on the incoming materials, synthesis method and assembly process, and manufacturing environment, a variety types of impurities may be introduced into the final battery product that impact the product performance.
In general, impurities can be categorized into four main types, namely particulate impurities, elemental impurities, organic impurities, and chemical impurities. , Particulate impurities are foreign objects that are introduced from the raw materials, manufacturing process, or environment, including particles and fibers. These impurity particles include metallic particles (e.g., Zn, Fe, Ni, Cr, Cu, stainless steel) and nonmetallic particles (e.g., SiO2, ZrO2). Fiber impurities refer to those polyester or cotton materials introduced from clothing, packaging materials, or filters from the environment. Particulate impurities adversely impact battery performance via internal resistance increase, localized defects, and dendrite growth, potentially causing short circuit and even thermal runaway. Elemental impurities consist of both metallic and nonmetallic elements in ionic form. These impurities can be either foreign elements introduced during the manufacturing process or excess quantities of elements beyond the expected levels. Elemental impurities impact battery performance via various mechanisms, including dendrite formation (Fe, Cu), enhanced corrosion (Cr, Cl), and the promotion of side reactions and byproduct formation (Mn, S, F). Organic impurities are compounds arising from solvent residue, binder, and electrolyte decomposition or other unwanted organic materials used in the manufacturing process. This type of impurity tends to interfere with electrochemical reactions, which potentially leads to reduced capacity and lower efficiency. Chemical impurities represent a broader category encompassing a wide range of chemical contaminants, including acid or alkali residues, phosphates, oxides, and moisture content. The presence of chemical impurities primarily drives side reactions, resulting in electrochemical instability, accelerated degradation, and a reduced cycle life.
To mitigate the impact of impurities in battery materials and the battery manufacturing process, a comprehensive strategy encompassing both control and monitoring measures should be implemented. From a control perspective, this includes minimizing the introduction of impurities from incoming materials by utilizing high-purity raw materials, maintaining a strictly controlled manufacturing environment to reduce environmental contamination such as moisture, dust, and foreign particles, and enhancing chemical processing steps, including purification and drying. Additionally, applying preventive coatings on metal parts that come into contact with materials or replacing metal parts with nonmetallic parts can reduce the introduction of metallic particles due to wear and tear. For the monitoring aspect, adopting both advanced analytical in-line (optical detection, X-ray imaging) and off-line techniques (e.g., optical microscopy, SEM-EDS, ICP-OES, ICP-MS, microCT, FIB-SEM) to detect and monitor impurities from incoming materials to the coating and cell assembly processes to final failure analysis for root cause analysis is essential. ,,− Figure shows an example of an impurity control procedure from incoming materials quality control to failure analysis via various imaging characterization approaches.
6.
In-line and off-line imaging characterization methods for impurity detection in battery manufacturing. (a) Incoming materials quality control for metal impurity identification in raw materials utilizing automated SEM and energy-dispersive spectroscopy. Reproduced with permission from ref . Copyright 2023 Nature Publishing Group. (b) In-line process quality control via an optical detection system. Top figure: Measurement setup of an optical detection system with a camera and two lights at different angles to the camera. Bottom figure: Various defect types for the electrode coating from a pilot-scale coater. Defects include agglomerates, cracks, contaminations, pinholes, slips, stripes, and microcompression. Reproduced from ref under the terms of the CC-BY 4.0 license. (c) Correlative imaging analysis from micro-CT to laser PFIB/SEM to identify the impurities within a pouch cell. In the current example, cathode particles mixed into the anode layer indicate cross-contamination issues in the manufacturing environment. Adapted from Thermo Fisher Scientific and Waygate Technologies.
3.2. Equipment Manufacturing
Manufacturing battery materials or components relies on many kinds of equipment, including characterization and data processing. For example, to make competitive synthetic graphite, a high temperature of at least 3000 °C must be achieved, which, as mentioned earlier, is about half the temperature of the surface of the sun. Many material properties at such an extreme temperature become unknown, i.e., vapor pressure, thermal and electrical conductivity, heat capacity, strength, and so on. The questions remaining to be answered include but are not limited to (1) Can graphite be produced in alternative ways with lower temperatures? (2) Can we recover the heat wasted? (3) Can plasma or induction be used to reduce the manufacturing cost of broad battery materials? and (4) Is there a better way to reduce the carbon footprint during materials production? In addition, to meet the soaring demand for graphite (and other materials), current suppliers may need to expand their production capacity by installing new generations of furnaces that will effectively address the issues of safety, reliability, and smart control in equipment design.
Not only does equipment manufacturing need to meet the different requirements of the manufacturer, such as space and payload, but reduction of the carbon footprint needs to be considered as well, saving energy and thus cost for materials production through simplifying the manufacturing process. For example, the state-of-the-art synthesis of NMC cathodes is through a precipitation method, followed by multiple steps of calcination, washing, and annealing (Figure ). A considerable amount of sulfate salts and alkaline solutions are used during this process, which also generates a significant amount of wastewater, creating an environment impact. Plasma technology is reported to simplify NMC manufacturing by skipping the coprecipitation step and avoiding the use of sulfates. Nitrate-based salts, such as LiNO3, Ni(NO3)2, Mn(NO3)2, and Co(NO3)2, can be directly mixed and heated quickly to form desired NMC cathodes using a microwave plasma process. The plasma technology is reported to minimize energy use and water consumption and eliminate solid/liquid waste production. A detailed cost analysis and comparison between the continuous synthesis process and the plasma approach is still needed, because NMC precursors, e.g., Ni0.8Mn0.1Co0.1(OH)2, are already commercialized, with a low cost of around $11.5/kg.
7.
Illustration of the state-of-the-art synthesis of NMC cathode materials. Reproduced from ref under the terms of the CC-BY 4.0 license.
Solid-state batteries are another example that require more innovations in engineering and equipment design. Although some similarities can be found in the electrode processing and cell assembly for LIBs, more challenges exist in scaling up the solid-state batteries with minimum defects on large format cells and consistent quality control. For example, different fabrication approaches (Figure ) can be adopted for making solid-state electrolyte membranes, free-standing or coated directly onto the electrode. The fabrication process of solid-state membranes or batteries needs to be conducted in a strictly controlled environment with rigorous requirements on cleanliness and humidity control because of the sensitivity of electrolytes, especially sulfides. Every single defect in the separator membrane may become a hot spot that leads to an internal short-circuit later in the solid-state batteries. A similar level of cleanliness also applies to the equipment, which needs enhanced durability when processing the hard oxide particles. Due to the zero-fluidity nature of solid-state electrolytes, minimizing porosity within the separator membrane (and within cathode) is critical but challenging for some of the electrolytes, such as the oxide-based ones. A sintering process with reduced temperatures aided by a sintering agent is reported to help, but the potential contamination to the crucibles and equipment raises the need for developing equipment dedicated for solid-state battery manufacturing with reduced energy consumption and contamination.
8.
Fabrication of solid-state electrolyte membranes as separators. Reproduced with permission from ref . Copyright 2018 Elsevier.
New equipment to recycle spent batteries is also needed. For example, new equipment or tools are needed to improve the ability to identify, sort, and characterize materials to facilitate recovery and recycling of these materials into new products for a circular economy.
3.3. Fast and Reliable Materials Validation
Scaled materials or components need fast validation to provide feedback to the production line to improve the manufacturing efficiency. Efficient and quick testing has become extremely important for this purpose. Half-cells using lithium metal as the anode are very helpful to assess either cathode or anode materials in terms of their usable capacities. However, to effectively evaluate their cycling stabilities, full cells are needed unless the assessment is for lithium metal anode. For example, freshly manufactured graphite- or silicon-based anodes need to be coupled with an already known stable cathode to understand if the long-term cycling stability, fast charging capabilities, etc. are consistent with targeted performances. Similarly, newly produced cathode materials need to be matched with stable graphite anodes to check the stability of both the cathode itself and the CEI layer. These seemingly very straightforward requirements are overlooked frequently in the literature, where Li metal is always applied as the counter electrode for evaluating either anode or cathode materials, hiding the true properties of the materials or their interfaces of interest. The electrochemical performance of any electrochemical cell is determined by the slowest step or worst component. Assuming the electrolyte, interphase, separator, etc. are all good, the observed properties are usually dominated by the worse electrode. To effectively assess electrode materials, the counter electrode needs to be sufficiently good, at least better or more stable than the one being assessed. In addition, depending on the specific use of the batteries to be deployed, the testing conditions of the scaled materials must be relevant for their practical applications. Figure displays the different opportunities arising during the process of materials scale-up and how the scaled materials are validated first in small coin cells, followed by further testing in realistic LIBs with specified capacity, energy, or power to ensure quality consistency. Protocols for fast coin cells testing need to be developed based on the ultimate application scenarios so that the results are helpful to estimate the materials’ properties in realistic batteries. ,
9.
Research cycles in small-scale laboratory synthesis and large-scale production. Reproduced with permission from ref . Copyright 2023 Nature Publishing Group.
3.4. Electrode Processing
Electrode fabrication is a crucial step in battery manufacturing as it impacts both the quality and cost of the batteries. , There are mainly two techniques employed for electrode manufacturing: dry or wet processing, each with its distinct advantages and challenges.
Wet electrode fabrication is the conventional and most widely adopted method in modern battery industries. This process involves mixing active materials, conductive additives, and binders with a solvent to create a homogeneous slurry. The slurry is then coated onto a current collector, such as aluminum or copper foil, followed by energy-intensive drying and solvent recovery steps. One of the main benefits of wet processing is its ability to achieve uniform coating thickness and electrode structures, which are essential for optimal battery performance. However, this method poses significant challenges, mostly related to the use, removal, and recovery of hazardous solvents such as N-methyl-2-pyrrolidone (NMP). Efficient solvent recovery and reuse, typically achieved through distillation, can mitigate some environmental impact and reduce processing cost. Sometimes, binder migration and slurry instability also happen during the wet coating process, leading to inhomogeneous distribution of materials.
The solvent-free dry process provides an alternative coating method by eliminating the need for solvent drying and recovery steps, thereby improving efficiency and saving energy. This technique usually involves the use of a dry powder formulation, calendared onto the current collector under high pressure to form an electrode layer. While conventional dry methods rely on mechanical mixing, they face issues with uniform dispersion and require a compatible binder and precise control of compaction forces. Dry extrusion helps with the dry coating process by offering superior mixing through controlled temperature and pressure. One advantage of applying dry processing is its ability to fabricate thick electrodes that traditional wet coating cannot achieve. However, the impedance of dry-processed electrodes is usually higher than that of conventionally coated ones because of the different types of binders used for dry coating, sacrificing some of the capacities packed in the thick electrodes.
The choice between dry and wet electrode fabrication methods should balance the specific requirements of the battery application, available manufacturing infrastructure, and environmental impact. For traditional battery materials, the traditional wet coating process still dominates due to the production quality and speed. For solid-state electrolytes or sulfur electrodes, dry processing may become very helpful, since it eliminates the necessity to expose those materials to solvents or moistures.
3.5. Characterizations in Battery Manufacturing
3.5.1. Characterizations for Quality Control
Quality control is critical in battery manufacturing. It assures battery reliability and safety and enables consistent performance via monitoring multiple parameters through the whole production cycle, from raw materials quality check and in-line process control to end-of-line testing. Characterization techniques, including imaging techniques (SEM, optical microscopy, FIB-SEM, laser, X-ray radiography, X-ray computed tomography), spectroscopic techniques (Raman, XRD, FTIR, ICP-OES), mass spectrometry techniques (GC-MS, ICP-MS), thermal techniques (TGA, DSC), and electrochemical techniques (EIS, cycling, four-point probe), both at nearline and in-line enable a holistic approach to monitor physical, mechanical, chemical, and electrical properties from battery materials to final cells.
Recently, the control of particulate impurities, particularly metal particles, has gained increased attention among materials suppliers, battery manufacturers, and original equipment manufacturers (OEMs) due to their potential to significantly affect battery lifespan and safety through side reactions, dendrite growth, and short circuits. Therefore, from materials synthesis to the final cell assembly process, stringent control and monitoring of the metal particles is required. For instance, in the process of LiFePO4 manufacturing, metal particle impurities primarily originate from three major sources: (1) the byproducts such as FeP, Fe2P, or even pure Fe metal formed when LFP is synthesized under a reduced atmosphere at high temperature (600–700 °C) with incomplete reaction, (2) the mixing and grinding processes that introduce metal particles due to the wear and tear of the metal parts that directly touch the materials, and (3) the floating metal particles in the air carried by operators or generated from the particles released from machinery within the factory. The corresponding mitigation strategies to control impurities include optimizing the synthesis process to minimize byproduct formation, applying coating layers (e.g., PTFE) on components such as the inner tank of mixers and grinding machines, incorporating magnetic separators or meshes into the process, and maintaining strict operational procedures to control the manufacturing environment. When moving to the cell assembly process at cell manufacturers, the introduction of metal impurities is then mainly from incoming materials, multiple assembly processes, including mixing, coating, calendaring, slitting, and welding, and the manufacturing environment (Figure a). Similar mitigation plans including preventive coating on the metal parts and strict control of manufacturing environment have been adopted.
10.
Particle impurity quality control in battery production. (a) Critical steps in the battery manufacturing process that introduce metal particle impurities. (b) Automated SEM-EDS analysis of the magnetic and nonmagnetic impurities collected from a battery manufacturing line. (c) Failure analysis of the separator from a failed battery cell via Cryo-FIB-SEM. Adapted from Thermo Fisher Scientific.
The industry complies with the technical cleanliness standards from the automotive sector, namely, VDA 19.2, to address particle contamination detection. Recently, the publication of GB/T 41704-2022 has provided specific guidelines for detecting metal impurities with a focus on magnetic impurities in cathode materials. Both magnetic metal particles (Fe, Cr, Ni) and nonmagnetic particles (Cu, Zn) are of significant concern and require precise detection methods. Given the critical importance of particle morphology and compositional information for impurity control, SEM and EDS, equipped with automation, are extensively utilized for off-line impurity detection. These techniques are employed to monitor the manufacturing process and generate quality control reports.
Due to the intrinsic physical properties of the two particle types, sample preparation methods differ when sampling incoming materials to final products for impurity analysis. Magnetic particles can be concentrated using a magnetic field, whereas nonmagnetic particles are primarily concentrated using a sieving method. The sieving method separates particles solely on the basis of size, which increases the likelihood of missing impurity particles and impacts the reliability of the results. For samples that need to be monitored in the manufacturing environment, industrial standard filters (47 mm diameter) are positioned at various locations within the manufacturing site to collect particles. Figure b illustrates SEM-EDS results of metal impurity particles collected from the manufacturing process. Morphological parameters and elemental information assist quality engineers in monitoring the cleanliness of the manufacturing environment and potentially tracing the source of impurities. If the impurity is not well controlled during the production process, it will stay as a contaminant in the final product, resulting in potential safety concerns. Figure c shows an example of failure analysis where a copper dendrite has been observed penetrating through the separator that causes cell short circuit and failure.
While off-line methods such as SEM-EDS are effective tools for actively monitoring impurities in battery manufacturing, offering both high-resolution imaging and accurate chemical composition analysis, their throughput is limited in covering the full sample volume, even with automation. Additionally, SEM-EDS is a destructive method, which restricts its applicability to access samples already in the manufacturing process. Consequently, there is a demand for nondestructive and in-line measurement methods.
3.5.2. In-Line Metrology in Battery Manufacturing
Scaling cell production from research and development (R&D) to high-volume manufacturing presents several challenges. During the R&D phase, processes are typically optimized on a small scale, where meticulous attention to detail and manual adjustments ensure high-quality outcomes. However, transitioning to high-volume manufacturing necessitates consistent and repeatable processes, which can expose variations and inefficiencies that were not apparent on a smaller scale. Issues such as maintaining uniform coating thickness, ensuring material homogeneity, and achieving precise alignment and layering become more pronounced. Additionally, high-volume production requires robust quality control systems to detect and rectify defects in real time, minimizing waste and ensuring conformance to specifications.
In-line metrology has emerged as a critical quality component, particularly in the context of cathode and anode coating processes. Uniformity and consistency of active material loading in the cathode and anode are among the most critical quality attributes determining the overall performance, energy density, and lifespan of batteries. The precise application of active materials in uniform, defect-free layers is crucial for maintaining the correct electrochemical balance in a battery cell. Variations in coating thickness, density, or composition can lead to a reduction in capacity, a shorter lifetime, and even safety hazards.
During the electrode coating process, in-line metrology tools that provide continuous monitoring of the surface defects, thickness profile, and mass loading are essential. High-resolution machine vision inspection cameras are used extensively on electrode coating lines to monitor the correct position of coating on the foil and to detect defects such as cracks and flaking. For mass loading measurement, traditionally, scanning noncontact gauges have been the primary technique used to measure the profile uniformity and thickness of electrode coatings. As shown in Figure a, by moving point sensors, such as confocal chromatic sensors for measuring thickness, or beta-ray and X-ray sensors for measuring the loading profile, back and forth across the electrode production line, scanning gauges provide high-precision measurements. This technology has been used for several decades in flexible film and metal sheet production.
11.
(a) Traditional noncontact scanning gauge with a single-point sensor. The resulting data represents a zigzag sampling path across the electrode as the coated foil moves through the process, typically capturing <5% of the electrode. (b) In-line mass profilometry maps the active material loading on the entire electrode. (c) Quality parameters and examples of coating defects that require 100% analysis of electrode coatings in battery manufacturing. (d) In-line metrology system configuration for a double-sided cathode coating line, which requires two synchronized in-line mass profilometry analyzers. Inset shows mass profilometry mapping of an NMC532 cathode as an example. Used with permission of Thermo Fisher Scientific, the copyright owner.
While well established, scanning gauges have important limitations. Point sensor scanning across the moving electrode foil in the coating process samples only a small fraction of the total production. Critical defects can be missed (Figure c). Additionally, scanning across the foil can take several seconds, during which tens of meters of nonconforming electrode can be produced, resulting in costly scrap.
The need to improve production yield and the potentially severe impact of in-field failure of battery cells call for new approaches. An emerging advanced metrology technique called in-line mass profilometry (Figure b) can analyze the mass loading across the entire electrode in real time and at full production speeds. Figure d shows an example system configuration of a double-sided cathode coating line, where two in-line mass profilometer analyzers are positioned before and after the dryers on each side to provide real-time quality assurance by analyzing 100% of the coated area. Capturing a complete side-to-side loading profile every millisecond, this new technology allows the user to detect previously invisible defects and make corrections to the process much faster than previously possible.
4. Digitalization of Battery Manufacturing
Digitalization is transforming battery manufacturing by enabling an accelerated scale-up of new innovations to build safe, energy-dense, and affordable batteries. , By integrating emerging digital technologies, such as digital twins, artificial intelligence (AI), big data, the Internet of Things (IoT), and automation, manufacturers gain a competitive edge through improved production efficiency, resource management, and closed-loop optimization. It has been reported that tens of millions of dollars could be saved annually using digital solutions for a gigafactory capable of 40 GWh production capacity.
Current efforts in production lines involve sensors coupled with machine learning (ML) algorithms to improve quality standards by identifying defects in electrode coating and early elimination of substandard cells. , Many defects arising from manufacturing variability, such as cathode overhang and tab weld failure, go unnoticed during electrochemical tests. In these scenarios, computer-vision-aided computed tomography scanning can reveal those defects and enhance cell quality metrics. In the context of battery testing, the application of data-driven methods has significantly shortened cell formation and aging studies by accelerating the test protocols and facilitating more rapid sorting algorithms. − Overall, improvements in the efficiency of resource management and a lower footprint via digital manufacturing can make a considerable impact when building gigafactories.
4.1. Digital Twin
In recent years, the concept of digital twins has gained considerable interest from academia and industry, as it eliminates the need for trial-and-error techniques and facilitates intelligent manufacturing process. As shown in Figure , battery digital twins are cyber-physical systems created based on detailed mapping of physical assets (raw materials, cells, and manufacturing process) using sensors. A digital framework that constitutes data collection methods, cloud computing, and several ML algorithms and physics-based simulations also interacts with physical assets through actuators to deliver data-driven and simulation results. This two-way communication promotes real-time monitoring and continuous updates of the digital twins. Finally, human integration in manufacturing will bridge the physical and digital spaces by enabling creative solutions, identifying anomalies, troubleshooting, ensuring the accuracy of data acquisition, and ultimately optimizing the digital twin feedback loop. The streamlined approach of battery digital twins will be beneficial for process optimization, property predictions, virtual simulation of material flow, and scheduled maintenance. The ARTISTIC project, funded by the European Research Council, underscores these use cases by utilizing physics-based models to simulate particle movements in the slurry during coating, drying, and calendaring processes and ML to enhance the speed and accuracy of these simulations by comparing them against experimental measurements. Notably, the majority of work on battery digital twins has focused on improving the efficiency of process management systems − and less on materials and cell design. , Furthermore, the scope and interconnection among digital twins across multiple steps in battery manufacturing are not yet fully understood.
12.
Schematic representation showing the concept of a digital twin for battery manufacturing. The communications between different layers, shown on the left, occurs via sensors, actuators, and human feedback.
4.2. Data Management
Data collection and selection strategies form the foundation for designing an accurate digital twin. Various strategies can be adopted for data measurement at various stages of battery manufacturing facilitated by sensors, optics, and gauges. At present, sensors are used at the systems level, with the primary purpose of calibration and safety. Future advancements in intelligent sensors and actuators hold potential for making data-driven decisions. However, challenges remain to address the noise generated from data collection and its propagation in the model. Probabilistic models that account for inherent variability have been found to be advantageous, leading to reliable predictions. Additionally, the heterogeneous data collected from different sources must be modified to a unified format for future use cases. The following stages after data collection involve data storing in a warehouse and data mining for future analysis. Continuous data-driven analysis performed within digital space yields optimized parameters that are then fed back to the physical assets, creating a closed-loop system that enables the machines to self-adjust and achieve the desired output. However, human intervention is currently required to implement these digital results. To reach complete digitalization, future work should emphasize developing an automated feedback loop at either the component or system level for a continuous information exchange. Another challenge to be addressed is the interoperability of different hardware and software, as well as the standardization of key metrics and measurement frequency.
4.3. Machine Learning Modeling
The extensive data generated from battery manufacturing make ML algorithms well-suited for predictive analysis, opening new avenues for accelerating battery innovation. Several recent reviews have highlighted the use of these models in materials informatics and cell production. − Studies − have demonstrated the ability of ML models to predict electrode properties such as porosity, thickness, and mass loading and final cell parameters using slurry properties as input. ML models also excel in predicting the nonlinear degradation patterns of batteries, resulting in enhanced safety and reliability (Figure a). , To date, most work has focused on employing ML for the prognosis and diagnosis of battery performance, creating a gap in utilizing data-driven methods for battery design, which often relies on physics-based modeling and simulations.
13.
Graphical representation showing the application of AI and autonomy in battery manufacturing. (a) Capacity degradation over cycle life (left) can be predicted (right) using data-driven models. Reproduced with permission from ref . Copyright 2019 Nature Publishing Group. (b) Automated search system formulating a new electrolyte. Reproduced with permission from ref . Copyright 2022 Elsevier. (c) Autonomous robot performing coin cell assembly in a glovebox. Reproduced from ref under the terms of the CC-BY 3.0 license. (d) Autonomous defect detection during electrode fabrication (colors indicate different defects detected). Reproduced with permission from ref . Copyright 2022 Wiley-VCH.
A few recent reviews have outlined the success of hybrid models that combine the predictive power of ML and high accuracy of physics-based models to evaluate battery electrode design and failure mechanisms. , A hybrid framework has been shown to reduce the number of experiments for optimizing battery electrode design. The robust nature of these models makes them ideal for advancing next-generation electrodes in lithium–sulfur, lithium–metal, and micro batteries. Similarly, these models have shown promising results in battery degradation modeling by capturing the underlying physics of electrochemical behavior along with rapid predictions. , Based on ML and physics integration, Aykol et al. showed different hybrid model architectures to improve lifetime predictions. However, notable challenges regarding hybrid models include the lack of industry-relevant data for training ML models and a disconnect between simulations and real production complexities.
4.4. Model Interpretability and Human Understanding
Deep learning models are complex and often lack transparency to users. Therefore, incorporating Explainable AI (XAI) or Interpretable ML (IML) provides meaningful insights to make informed decisions. Explainability refers to understanding the cause and effect of ML predictions in human terms, and interpretability is understanding the inner mechanics of the ML model, such as weights and biases. XAI/IML techniques offer the benefits of understanding predictions on unseen data in battery production and management. Their study indicated that most of the reported literature on model interpretability focuses on LIBs. Many XAI/IML techniques, e.g., Partial Dependence Plots (PDP) and surrogate models and SHapley Additive exPlanations (SHAP), are model- and chemistry-agnostic, and the knowledge acquired can be transferred to chemistries beyond lithium.
A fundamental approach in this domain involves estimating the impact of each feature on the final prediction. Among several packages, SHAP is widely used in the battery community as it provides local (individual prediction) and global (across multiple predictions) feature contribution scores along with powerful visualization tools. When discovering novel electrode materials, SHAP analysis can reduce the physicochemical feature space by nearly 90%, based on each point’s contribution to electrode voltage predictions. Using these scores, ML models with prior knowledge can be developed to narrow down the search for electrode materials from thousands to just tens of them. SHAP can also be used to analyze complex aging phenomena in batteries and understand the relationship between cycling conditions and lifetime predictions, for instance, how the degradation of cathode and anode electrodes contributes to overall cell resistance. These insights are crucial for understanding in-depth the behavior of internal cell components, despite having data only at the cell level.
In addition to feature contributions, calculating feature interactions quantitively using Friedman’s H-statistics provides a valuable measure. Since all the stages in battery manufacturing are interconnected with sequential data transfer, feature interactions will be essential. For example, the final electrode thickness and electrolyte quantity measured from two different stages may determine the final cell characteristics. Hence, these model interpretability approaches can potentially optimize battery manufacturing by finding a balance between performance and safety without compromise.
4.5. Toward Autonomous Production
State-of-the-art industrial battery production is largely automated. However, the use of AI and data-driven decision-making is still far from common. Recent efforts are focused on developing intelligent cyber-physical systems, driving a paradigm shift toward fully autonomous operations. Notably, the integration of advanced ML models with robotics can optimize complex multivariable problems, such as those encountered in R&D battery electrolyte formulation (Figure b). , Compared to traditional methods that take up to months to identify a suitable electrolyte within a given design space, autonomous optimization expedites the process by 6 times in a laboratory setting. At a pilot and production scale, autonomous systems can be effective in identifying and reducing manufacturing variability-induced defects such as misalignments during electrode fabrication and cell assembly, and their closed-loop optimization capabilities further enhance precision (Figure c,d). , Beyond battery innovation, automated disassembly systems are capable of high-quality extraction of recycling materials from battery packs and reduces the risks associated with manual battery recycling methods.
AI and autonomous systems perform human-like tasks efficiently. However, human intelligence remains crucial in scientific breakthroughs and solving unexpected problems that often rely on collaboration and communication. Battery production is a highly complex and interconnected process that requires experts from multidisciplinary domains to collaborate, bringing their creative thinking and hypothesis generation. The future of battery innovation lies not in the replacement of human ingenuity but rather in a synergistic relationship among humans, AI, and autonomous systems.
5. Recycling
The growing demand for LIBs has raised concerns about the sustainability of critical raw materials such as nickel, cobalt, and lithium, which have been well discussed in a few articles. , With millions of EVs already on the road and expected growth of batteries to improve grid resilience, the need for a robust battery recycling infrastructure is more urgent than ever.
EV batteries have a lifespan of about 10–15 years, meaning that a substantial number of early-generation batteries will soon be retired, especially those with lower nickel content. However, today’s battery chemistry has evolved significantly, with newer formulations favoring higher nickel content, reduced cobalt dependency, and in some cases, entirely different materials, such as LiFePO4. This shift presents a challenge for recycling, since older batteries may not align with current manufacturing needs. Despite these differences, recycling remains crucial for reducing the reliance on mined raw materials, minimizing environmental impact, and reintegrating valuable components into the supply chain. Additionally, before reaching the recycling stage, many retired EV batteries can still serve second-life applications in less demanding settings, such as grid storage or backup power for data centers. This approach extends battery lifespan, enhances energy security, and delays waste generation, contributing to a more sustainable battery ecosystem.
While LIB recycling is a pressing concern today, looking ahead, the emergence of next-generation battery technologies such as sodium-ion and solid-state batteries will introduce new challenges and demands in recycling. These alternative chemistries are being developed to improve safety, cost-effectiveness, and resource availability, but they also require tailored recycling methods that differ from the existing LIB recycling infrastructure. Current recycling technologies, including pyrometallurgy and hydrometallurgy, may not be directly compatible with new material compositions, necessitating further innovation in recycling processes. Additionally, direct-recycling methods, , like molten-salt direct recycling, which allow flexibility in tuning composition and microstructure, could play a key role in adapting to future battery chemistries. Using new salt Li2O to recycle spent polycrystal NMCs directly into high-performance single crystals provides new insights on direct recycling of cathode materials, especially for the scraps from the production line, which is nontrivial.
As the battery industry continues to evolve, proactive research and investment in next-generation recycling strategies will be essential to ensuring a closed-loop system for all battery types, reducing the environmental impact, and securing long-term material sustainability. Similar to battery and materials manufacturing, cost will still be the major criterion determining when and where the recycling technology will be accepted by the market. If the cost of recycling cannot be reduced to make the recycled elements cost-competitive with freshly mined ones, it will not be utilized by industry, which highlights the importance of cost-oriented fundamental research in recycling as well.
6. Workforce Training for Battery Manufacturing: From Operators to Ph.D.s
The continuous growth of EVs places pressing challenges globally on battery manufacturing and the supply chain. Currently, the U.S. has 59 GWh of cell production capacity annually, with the majority of the required components and raw materials being imported from Asia. The U.S. would require more than 1,000 GWh battery production annually to meet the goal of 100% domestic manufacturing of EVs by 2030. Even if minerals and production facilities were fully available, a shortage of skilled labor is a seminal challenge in the achievement of even this single production goal, let alone the myriad other uses of LIBs.
Legacy automotive manufacturing skillsets emphasize mechanical engineering and machining capabilities, instead of knowledge of battery chemistries, electronics, and industrial engineering which are at the center of battery workforce needs. Transitioning to battery production has proven difficult despite significant federal incentives, due in part to the lack of available education, in part to a lack of societal understanding of the need, and in part to a mining and manufacturing “image” problem. Addressing these challenges requires a national strategy that addresses targeted training, industry–academic collaboration, and the development of standards tailored to battery production. Investment in R&D and process optimization is crucial to improving manufacturing yield and competitiveness. Tailored and consistent, peer-reviewed (i.e., accredited), and highly accessible education and training that integrate case studies demonstrating how to apply textbook knowledge to solve manufacturing problems may inspire more individuals to apply their talents within this field.
There are certainly positive examples of programs designed to educate and train a future battery workforce, such as New Energy New York’s “Battery Academy” or the Center for Energy Workforce Development’s “Energy Industry Fundamentals”, but a more widespread and cohesive effort is required. Industry recruitment must expand and be promoted, as workers are in short supply, and many federally funded industrial efforts must be more connected to one another to prevent gaps and overlaps. To support this objective, a greater focus is needed on opportunities across the educational spectrum, from entry-level training in the basics of workplace safety to the advanced skills of highly educated engineers. More students in educational programs leading to future careers in the battery industry will also necessitate the identification and recruitment of faculty with the appropriate skills and experience to educate the growing body of battery workers.
In recognition of these new realities, industry, government, and academic organizations have begun to join together to create opportunities for education for both students and educators. One of the most prominent programs to come from such a collaboration is the DOE’s Battery Workforce Initiative (BWI). The BWI seeks to attract fresh talent to the battery manufacturing industry. By collaboration with industry leaders, the initiative aims to pinpoint the essential competencies required for battery manufacturing roles. Insights gathered from experts will inform the standardization of national training to meet skill requirements while addressing gaps in battery manufacturing. Industry needs all levels of workers, from operators to Ph.D.s, to work on the production line. “While many programs already exist to train workers for battery manufacturing, there is a significant mismatch between the skills attained through classroom or lab training and the skills required on the job.” The BWI provides a good start at filling that gap by describing required classroom instruction, outlining opportunities for experiential learning, and suggesting mentorship opportunities to train individuals in the battery manufacturing industry.
The New Energy New York (NENY) “Battery Academy” stands as one of the nation’s most advanced and comprehensive battery training programs. Since its pilot launch in early 2023, NENY programs have trained over 1,000 individuals for careers in the battery industry. The NENY Battery Technician program encompasses a hybrid of online, virtual reality, and hands-on lab learning, comprehensive learning guides, instructor-led office hours, and robust support services. Moreover, NENY’s K–12 programming achieved the New York Department of Labor’s cluster approval for a pioneering two-year learning curriculum, empowering graduates with diplomas, nationally recognized microcredentials endorsed by Binghamton University, and practical experience in high-demand careers. With funding for workforce development primarily focused on high placement outcomes, this program stands as a blueprint to the nation on the return of investment when investing in youth as a part of the workforce ecosystem.
There are also programs at Battery Belt institutions that partner to address future workforce requirements. Greenville Technical College, Trident Technical College, and Spartanburg Community College established a consortium titled “Revolutionizing Electric Vehicle Education”, resourced by the National Science Foundation and dedicated to advancing and exploring the utilization of virtual reality (VR) and augmented reality (AR) educational resources to bolster EV and battery manufacturing. Immersive digital trends like mixed reality are being explored to train workforces in laboratory and pilot lines. These hands-free devices empower battery engineers and researchers with real-time data collection, access to digital twin, and guidance, thereby reducing errors in battery assembly and development.
7. Outlook
Battery and battery materials manufacturing present a unique opportunity for researchers and industry partners to work together to accelerate lab-to-market technology translation and establish a healthy and resilient supply chain. Cost-oriented fundamental research will become a priority when addressing the manufacturing challenges.
-
1.
Understanding and utilizing “impurities” to take advantage of “cheaper” and/or “dirtier” raw materials to produce similarly good battery materials not only is scientifically interesting but also helps diversify the battery supply chain. Effective monitoring and control of impurities from raw materials to final production will be critical for this purpose. Materials synthesis that can tolerate wide temperature ranges through innovations will save much energy and enhance consistent quality, thus saving manufacturing cost. Still, even for the conventional battery materials such as NMCs, LFP, graphite, and LiPF6-based electrolytes, there is much innovation space to help further reduce the manufacturing cost and carbon footprint during production through impurity control or impurity utilization, equipment design, as well as removal of toxic solvents. The upstream materials also needs scientific and engineering solutions to efficiently extract key elements through efficient separation. New ideas are also needed to concentrate Nickel II for nickel sulfate production for subsequent cathode materials manufacturing. It may be a good time to revisit the electrowinning process of copper and aluminum by considering the specifications in battery applications and develop a sustainable approach for supplying those inactive components for battery production. Reusing the scraps and recycling the valuable elements from spent batteries will further secure the supply chain stability, if the cost of battery recycling can be further reduced, which is achievable through materials and process innovations.
-
2.
Simplifying production process, instead of adding more steps for incremental performance improvement, will be the priority to explore manufacturing science. Decreasing the number of materials synthesis steps will ease the manufacturing process at a large scale and reduce the uncertainties introduced by those multiple steps. For example, washing is usually needed for NMCs to get rid of the residual lithium salts after calcination. An annealing process is therefore also needed to “restore” the structure of the washed cathode materials. It is worthy of further investigation to convert those lithium salt leftovers into buffer layers by co-sintering with other elements or oxide, thus removing the washing step while improving the materials’ performances. Alternatively, if the excessive amount of lithium salts becomes unnecessary for high-temperature calcination, the washing step will also be removed. Large-scale processing of defect-free solid-state electrolytes, especially in the separator membrane format, is still a big challenge, while materials-level innovations also need to be sought to identify practical approaches for stabilizing sulfide-based electrolytes in ambient environment or at least making them less sensitive to moisture.
-
3.
Streamlining the manufacturing process through digital twins will play an important role in enhancing production efficiency. With the fast development of sensors, gauging systems, etc., extensive amounts of data are generated from battery manufacturing process. Analysis and extraction of key information from those massive data will rely much on ML and AI. Successful implementation of ML models in battery production will make it possible to predict or even automatically adjust key parameter based on the property variations from different batches of materials to ensure the consistent quality of coating, loading, porosity, and tortuosity control. Experimental works are combined with advanced theoretical and computational approaches to digitalize battery cell manufacturing from process optimization, performance prediction, degradation analysis to visual simulation of materials flow, and scheduled maintenance, all of which are streamlined to enhance the manufacturing efficiency and reduce the cost. Virtual reality/mixed reality digital twins are also now designed for training purposes and to assist decision-making in the battery manufacturing process.
-
4.
A robust and diverse workforce is pivotal to meet the industry’s escalating demands, fostering inclusivity across backgrounds, ethnicities, and beliefs. Battery manufacturers not only need researchersthey also need technicians, operators, and so on. Training courses and curricula need to be developed and tailored for specific needs such as mining or the electrochemical industry in addition to classic battery-related training. The most effective approach is still through public–private collaboration, which enables a true understanding of the challenges that industry faces in hiring and developing a workforce and in accessing education and training programs that enable cultivation of a strong, effective, and enduring battery workforce.
Acknowledgments
This review article was drawn from the discussion of a manufacturing science workshop (September 2023), which was jointly organized by the University of Washington and Pacific Northwest National Laboratory (PNNL) and supported by the Advanced Materials and Manufacturing Technologies Office (AMMTO) of Energy Efficiency and Renewable Energy (EERE), U.S. Department of Energy (DOE). J.X. also thanks Letian Li, Remco Geurts, Bartlomiej Winiarski, and Haifeng Gao at Thermo Fisher Scientific and Tobias Neubrand at Waygate Technologies for the images used to create Figures and , and Alex Sammut at Thermo Fisher Scientific for helpful discussions on the workforce section. PNNL is operated by Battelle for the Department of Energy under contract DE-AC05-76RLO1830.
Glossary
Abbreviations
- AI
artificial intelligence
- AR
augmented reality
- ASSLB
all-solid-state battery
- BWI
Battery Workforce Initiative
- CEI
cathode–electrolyte interphase
- CVD
chemical vapor deposition
- DOE
Department of Energy
- DSC
differential scanning calorimetry
- EDS
energy-dispersive spectroscopy
- EIS
electrochemical impedance spectroscopy
- EV
electric vehicle
- FIB
focused ion beam
- FTIR
Fourier transform infrared spectroscopy
- GC
gas chromatography
- GWh
gigawatt-hours
- GWP
global warming potential
- HCE
high-concentration electrolyte
- ICP
inductively coupled plasma
- IML
interpretable machine learning
- LFP
lithium iron phosphate
- LHCE
localized high-concentration electrolyte
- LiAsF6
lithium hexafluoroarsenate
- LiBH4
lithium borohydride
- LIB
lithium-ion battery
- LiFSI
lithium bis(fluorosulfonyl)imide
- LGPS
Li10GeP2S12
- LiPF6
lithium hexafluorophosphate
- LiTFSI
lithium bis(trifluoromethanesulfonyl)imide
- ML
machine learning
- MS
mass spectrometry
- NENY
New Energy New York
- NMC
LiNi x Mn y Co z O2
- NMC111
LiNi1/3Mn1/3Co1/3O2
- NMC532
LiNi0.5Mn0.3Co0.2O2
- NMC622
LiNi0.6Mn0.2Co0.2O2
- NMC811
LiNi0.8Mn0.1Co0.1O2
- NMC90
LiNi0.9Mn0.05Co0.05O2
- OES
optical emission spectroscopy
- PDP
partial dependence plot
- PFIB
plasma focused ion beam
- PTFE
polytetrafluoroethylene
- SEI
solid–electrolyte interphase
- SHAP
SHapley Additive exPlanations
- SSE
solid-state electrolyte
- SPAN
sulfurized polyacrylonitrile
- TGA
thermogravimetric analysis
- XAI
explainable artificial intelligence
Biographies
Jie Xiao is currently a Boeing Martin Professor in Mechanical Engineering at University of Washington (UW) with a joint appointment as a Battelle Fellow at Pacific Northwest National Laboratory (PNNL). She has been leading research thrusts on both practical applications and fundamental study of energy storage materials and systems, spanning from microbatteries for sensors to advanced battery technologies for vehicle electrification and grid energy storage. Dr. Xiao has published more than 100 papers and holds 18 patents in the area of energy storage. She earned her Ph.D. degree in Materials Chemistry from State University of New York Binghamton.
Xia Cao is a Materials Scientist at Pacific Northwest National Laboratory (PNNL), specializing in battery technology. She earned her Ph.D. in Physical Chemistry from the University of Münster (Westfälische Wilhelms-Universität) in Germany. With over 14 years of experience, her research centers on developing advanced electrolytes and deepening the understanding of electrode/electrolyte interphases. Her work aims to improve the stability, safety, and performance of high-energy-density rechargeable batteries, including lithium-ion, lithium metal, and potassium-ion batteries.
Bernard Gridley is the Director of Research & Development at Anovion Technologies, a leading advanced materials business focused on North American production of synthetic graphite for lithium-ion batteries. Bernard is a seasoned technical leader and entrepreneur with 20 years of experience in energy storage, specializing in materials and process innovation and commercialization. He has held senior roles in several pioneering energy storage companies, where he led teams in research, development, and commercialization of advanced battery and ultracapacitor technologies.
William Golden is a director and owner of Borman Specialty Materials, a producer of manganese-based cathode material located in Henderson, Nevada. He is also a director and owner of Prime Metals & Alloys, a producer of nickel- and cobalt-based superalloys located in western Pennsylvania. He holds degrees from Princeton University, The London School of Economics and Political Science, and Fordham University.
Yuchen Ji is currently a postdoctoral scholar in Department of Mechanical Engineering at University of Washington. He obtained his Ph.D. degree from Peking University in 2024. His research interests focus on interfacial evolution and in situ characterizations in secondary batteries.
Stacey Johnson is the Director of Workforce and Economic Development at the University at Buffalo. Previously at Binghamton University, Johnson has been a driving force behind regional and national workforce initiatives in the battery and energy storage industry. As Director of Workforce Development for the U.S. EDA-funded New Energy New York (NENY) initiative and the NSF Upstate New York Energy Storage Engine, Johnson has led efforts that have trained over 1,500 individuals for careers in the growing battery and energy storage sector.
Dongping Lu is the Chief Scientist and Team Leader for the Battery Materials Research team at Pacific Northwest National Laboratory (PNNL). His research integrates fundamental understanding, materials discovery, and advanced processing techniques for energy storage and his current work focuses on the utilization of low-cost sulfur materials for high-energy, long-life batteries, spanning across liquid, Li-ion, and all-solid-state lithium–sulfur systems. Dr. Lu is an inventor with 11 granted and pending patents, and he has published over 70 peer-reviewed papers.
Feng Lin is a Professor of Chemistry and the Leo and Melva Harris Faculty Fellow at Virginia Tech. He is also jointly appointed in the Department of Materials Science and Engineering and affiliated with the Macromolecules Innovation Institute at Virginia Tech. Prior to joining Virginia Tech in 2016, Dr. Lin worked at QuantumScape Corporation as a Senior Member of the Technical Staff, and at Lawrence Berkeley National Lab as a Postdoctoral Fellow.
Jun Liu, is the Washington Research Foundation Innovation Chair in Clean Energy, Campbell Chair of Materials Science & Engineering, Professor of Chemical Engineering, and a Battelle Fellow at the Pacific Northwest National Laboratory (PNNL). Jun Liu’s main interest is developing fundamental principles to guide materials synthesis, characterization and application of advanced materials for energy, biomedicine and environment, development and deployment of new materials and technologies for electric vehicles, grid scale energy storage and modern communications. He has been ranked in the top one percent of highly cited researchers since 2014 (Clarivate Analytics).
Yijin Liu is an Associate Professor at UT Austin with a research portfolio that reflects nearly two decades of expertise in developing and applying X-ray characterization methods across diverse scientific fields. His work centers on energy storage materials, employing high-throughput experimental techniques and machine learning-assisted statistical analysis. Key areas of focus include battery manufacturing, safety, degradation, and failure analysis, all dedicated to advancing reliable, high-performance energy storage solutions.
Zhao Liu is currently Sr. Market Development Manager at Thermo Fisher Scientific. He holds Bachelor’s degree (2007) and MSc degree (2010) in Physics from Nanjing University, and Ph.D. degree (2015) in Materials Science and Engineering from Northwestern University. Zhao’s current focus is to investigate the analytical challenge of battery characterization in both battery R&D and manufacturing, develop solutions to meet the characterization needs.
Hemanth Neelgund Ramesh is a PhD student in Mechanical Engineering at the University of Washington, advised by Prof. Shijing Sun. His research focuses on the digital transformation of battery manufacturing using emerging AI tools. He completed his MS in Materials Science and Engineering at UW, where he completed his thesis on battery informatics using explainable machine learning.
Feifei Shi currently serves as an assistant professor of energy engineering in the John and Willie Leone Family Department of Energy and Mineral Engineering. Dr. Shi’s research interests focus on in situ diagnosis tools and protocols for renewable energy, manufacturing innovation for sustainable energy conversion, and storage systems and sustainable electrochemical-separation process for critical elements. The author of 55 articles (h-index 41) and one book chapter, she serves as the guest editor for Frontiers in Energy Research, Energy & Environmental Materials (EEM) and the editorial board of Energy Materials.
Jeremy Schrooten brings nearly 25 years of technical and business leadership experience to his role as the Vice President of Technology for Anovion. He is responsible for the Technology Center of Excellence that includes product and process research and development, customer technical support, and laboratory operations. Prior to this role, Dr. Schrooten served as Technical Director of Battery Materials for Pyrotek Inc., a U.S.-based global leader of engineered materials, where he led the company’s development and commercialization of lithium-ion grade graphite materials. Prior to joining Pyrotek, Dr. Schrooten held various technical management positions with a diverse set of companies including United Technologies, Angstrom Power, and Société Bic S.A. Dr. Schrooten has been awarded 47 patents, written numerous papers, and presented at many industry and technical conferences. Dr. Schrooten holds two Bachelor of Science degrees in Chemistry and Ceramic Engineering, and a Ph.D. in Materials Science and Engineering from Iowa State University.
Mary Jenkins Sims earned a commission as an Ensign in the U.S. Navy on 31 March 1989 and was the first woman in U.S. Naval history to be permanently stationed aboard a forward-deployed vessel. Now retired from the Navy, she is a member of the Energy Academic Group at the Naval Postgraduate School. With the support and backing of the Department of Defense (DoD), she has developed a strategic roadmap for the domestic battery workforce that is today informing the development of federal battery workforce policies. Dr. Sims continues her research into battery workforce issues throughout the supply chain and leads an advisory group of battery technology experts in advising the DoD and others on battery issues throughout the U.S.
Shijing Sun is an Assistant Professor at the University of Washington. Her research centers on autonomous materials discovery and design, emphasizing collaborative intelligence that integrates human expertise with robotics and artificial intelligence to advance clean energy innovation. Dr. Sun previously held a Senior Research Scientist position at the Toyota Research Institute and was a research scientist at Massachusetts Institute of Technology. She earned her B.A. in Natural Sciences and M. Sci. and Ph.D. in Materials Science at the University of Cambridge under Prof. Anthony K. Cheetham.
Yuyan Shao is an expert in the field of electrochemistry and materials science. He is a laboratory fellow at Pacific Northwest National Laboratory. He has been conducting applied and fundamental research on materials, chemistry and processes for energy storage and conversion technologies for transportation and stationary applications. His research has been focused on understanding electrochemical interfaces and developing high performance materials for batteries and energy storage, as well as hydrogen and fuel cells. He is an author of over 170 peer-reviewed publications and an inventor on 10 U.S. patents. He has been listed in Clarivate’s Highly Cited Researchers multiple times.
Alon Vaisman is a Senior Manager of Strategic Business Development in Thermo Fisher’s Production & Process Analytics Business Unit. In this role Alon leads strategic projects and collaborations with industry leaders and technology partners, focusing on the Clean Energy market segment. Alon’s background is in Mechanical Engineering and prior to joining Thermo Fisher in 2021 Alon held leading roles in product and application management at a global scientific instrumentation company focusing on particle characterization. Alon has over 20 years of experience in the Process Analytical Technology space, working in Pharmaceutical, Environmental and Advanced Materials sectors.
Jihui Yang holds the Kyocera Corporation Chair in Ceramic Engineering and is a Professor in the Materials Science and Engineering Department at the University of Washington (UW). Prior to joining UW in Fall 2011, he served as a Technical Fellow and Lab Group Manager at the GM Research and Development Center. His current research focuses on electrochemical energy storage, solid-state energy conversion, electrocatalysis, and the transport properties of quantum materials.
Stanley Whittingham is a distinguished professor of Chemistry and Materials S&E at Binghamton University (SUNY). He received the 2019 Chemistry Nobel Prize for his invention of the lithium battery at Exxon in 1972. He is a Fellow of the Royal Society and a member of the National Academy of Engineering. He continues to lead research efforts from fundamental science of energy storage devices to their commercialization. He presently leads efforts led by Binghamton University funded by the U.S. EDA BBBRC (NENY) and the NSF through their Engines Program.
Jie Xiao writing-original draft, writing-review and editing, conceptualization, supervision; Xia Cao writing-original draft; Bernard Gridley writing-original draft; William Golden writing-original draft; Yuchen Ji writing-review and editing, visualization; Stacey Johnson writing-original draft; Dongping Lu writing-original draft; Feng Lin writing-original draft; Jun Liu writing-review and editing; Yijin Liu writing-original draft; Zhao Liu writing-original draft, visualization; Hemanth Neelgund Ramesh writing-original draft, visualization; Feifei Shi writing-original draft; Jeremy Schrooten writing-original draft; Mary J. Sims writing-original draft, writing-review and editing; Shijing Sun writing-original draft; Yuyan Shao writing-original draft; Alon Vaisman writing-original draft; Jihui Yang writing-original draft; M. Stanley Whittingham writing-review and editing;
The authors declare no competing financial interest.
References
- Xiao J., Shi F., Glossmann T., Burnett C., Liu Z.. From laboratory innovations to materials manufacturing for lithium-based batteries. Nat. Energy. 2023;8(4):329–339. doi: 10.1038/s41560-023-01221-y. [DOI] [Google Scholar]
- Ziegler M. S., Trancik J. E.. Re-examining rates of lithium-ion battery technology improvement and cost decline. Energy Environ. Sci. 2021;14(4):1635–1651. doi: 10.1039/D0EE02681F. [DOI] [Google Scholar]
- Liu J., Bao Z., Cui Y., Dufek E. J., Goodenough J. B., Khalifah P., Li Q., Liaw B. Y., Liu P., Manthiram A., Meng Y. S., Subramanian V. R., Toney M. F., Viswanathan V. V., Whittingham M. S., Xiao J., Xu W., Yang J., Yang X.-Q., Zhang J.-G.. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy. 2019;4(3):180–186. doi: 10.1038/s41560-019-0338-x. [DOI] [Google Scholar]
- Porzio J., Scown C. D.. Life-Cycle Assessment Considerations for Batteries and Battery Materials. Adv. Energy Mater. 2021;11(33):2100771. doi: 10.1002/aenm.202100771. [DOI] [Google Scholar]
- Gutsch M., Leker J.. Costs, carbon footprint, and environmental impacts of lithium-ion batteries - From cathode active material synthesis to cell manufacturing and recycling. Appl. Energy. 2024;353:122132. doi: 10.1016/j.apenergy.2023.122132. [DOI] [Google Scholar]
- Kim H. C., Wallington T. J., Arsenault R., Bae C., Ahn S., Lee J.. Cradle-to-Gate Emissions from a Commercial Electric Vehicle Li-Ion Battery: A Comparative Analysis. Environ. Sci. Technol. 2016;50(14):7715–22. doi: 10.1021/acs.est.6b00830. [DOI] [PubMed] [Google Scholar]
- Pell R., Tijsseling L., Goodenough K., Wall F., Dehaine Q., Grant A., Deak D., Yan X., Whattoff P.. Towards sustainable extraction of technology materials through integrated approaches. Nat. Rev. Earth Env. 2021;2(10):665–679. doi: 10.1038/s43017-021-00211-6. [DOI] [Google Scholar]
- Zhou Y.. Lifecycle battery carbon footprint analysis for battery sustainability with energy digitalization and artificial intelligence. Appl. Energy. 2024;371:123665. doi: 10.1016/j.apenergy.2024.123665. [DOI] [Google Scholar]
- Bi Y., Xu Y., Yi R., Liu D., Zuo P., Hu J., Li Q., Wu J., Wang C., Tan S., Hu E., Li J., O’Toole R., Luo L., Hao X., Venkatachalam S., Rijssenbeek J., Xiao J.. Simultaneous Single Crystal Growth and Segregation of Ni-Rich Cathode Enabled by Nanoscale Phase Separation for Advanced Lithium-Ion Batteries. Energy Storage Mater. 2023;62:102947. doi: 10.1016/j.ensm.2023.102947. [DOI] [Google Scholar]
- Bi Y., Tao J., Wu Y., Li L., Xu Y., Hu E., Wu B., Hu J., Wang C., Zhang J. G., Qi Y., Xiao J.. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science. 2020;370(6522):1313–1317. doi: 10.1126/science.abc3167. [DOI] [PubMed] [Google Scholar]
- Louli A. J., Eldesoky A., Weber R., Genovese M., Coon M., deGooyer J., Deng Z., White R. T., Lee J., Rodgers T., Petibon R., Hy S., Cheng S. J. H., Dahn J. R.. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy. 2020;5(9):693–702. doi: 10.1038/s41560-020-0668-8. [DOI] [Google Scholar]
- Al-Bidry M. A., Azeez R. A.. Removal sulfur components from heavy crude oil by natural clay. Ain. Shams. Eng. J. 2020;11(4):1265–1273. doi: 10.1016/j.asej.2020.03.010. [DOI] [Google Scholar]
- Spackman I., Anderson C., Liu J., Xiao J., Lu D.. Optimizing high energy density sulfur cathodes: A multivariate approach to electrode formulation and processing. Energy Storage Mater. 2024;73:103727. doi: 10.1016/j.ensm.2024.103727. [DOI] [Google Scholar]
- Xiao J.. Understanding the Lithium Sulfur Battery System at Relevant Scales. Adv. Energy Mater. 2015;5(16):1501102. doi: 10.1002/aenm.201501102. [DOI] [Google Scholar]
- Xiao J., Anderson C., Cao X., Chang H.-J., Feng R., Huang Q., Jin Y., Job H., Kim J.-M., Le P. M. L., Liu D., Seymour L., Shamim N., Shi L., Sivakumar B.. PerspectiveElectrochemistry in Understanding and Designing Electrochemical Energy Storage Systems. J. Electrochem. Soc. 2022;169(1):010524. doi: 10.1149/1945-7111/ac4a55. [DOI] [Google Scholar]
- Ji Y., Yang K., Liu M., Chen S., Liu X., Yang B., Wang Z., Huang W., Song Z., Xue S., Fu Y., Yang L., Miller T. S., Pan F.. PIM-1 as a multifunctional framework to enable high-performance solid-state lithium-sulfur batteries. Adv. Funct. Mater. 2021;31(47):2104830. doi: 10.1002/adfm.202104830. [DOI] [Google Scholar]
- He B., Zhang F., Xin Y., Xu C., Hu X., Wu X., Yang Y., Tian H.. Halogen chemistry of solid electrolytes in all-solid-state batteries. Nat. Rev. Chem. 2023;7(12):826–842. doi: 10.1038/s41570-023-00541-7. [DOI] [PubMed] [Google Scholar]
- Salakjani N. K., Singh P., Nikoloski A. N.. Production of Lithium - A Literature Review Part 1: Pretreatment of Spodumene. Production of Lithium - A Literature Review Part 1: Pretreatment of Spodumene. 2020;41(5):335–348. doi: 10.1080/08827508.2019.1643343. [DOI] [Google Scholar]
- Vera M. L., Torres W. R., Galli C. I., Chagnes A., Flexer V.. Environmental impact of direct lithium extraction from brines. Nat. Rev. Earth Env. 2023;4(3):149–165. doi: 10.1038/s43017-022-00387-5. [DOI] [Google Scholar]
- Lang J., Jin Y., Liu K., Long Y., Zhang H., Qi L., Wu H., Cui Y.. High-purity electrolytic lithium obtained from low-purity sources using solid electrolyte. Nat. Sustain. 2020;3(5):386–390. doi: 10.1038/s41893-020-0485-x. [DOI] [Google Scholar]
- Choe G., Kim H., Kwon J., Jung W., Park K. Y., Kim Y. T.. Re-evaluation of battery-grade lithium purity toward sustainable batteries. Nat. Commun. 2024;15(1):1185. doi: 10.1038/s41467-024-44812-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Park K., Lee H., Noh Y., Kossowska D., Kwak K., Cho M.. Ultrafast fluxional exchange dynamics in electrolyte solvation sheath of lithium ion battery. Nat. Commun. 2017;8:14658. doi: 10.1038/ncomms14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, D. ; Asmussen, R. M. ; Kuo, L.-J. ; Hu, J. . Electrochemical lithium extraction for battery materials. U.S. Patent 17843392, 2022.
- Liu Y., Ma B., Lü Y., Wang C., Chen Y.. A review of lithium extraction from natural resources. Int. J. Miner., Metall. Mater. 2023;30(2):209–224. doi: 10.1007/s12613-022-2544-y. [DOI] [Google Scholar]
- Peltosaari O., Tanskanen P., Hautala S., Heikkinen E.-P., Fabritius T.. Mechanical enrichment of converted spodumene by selective sieving. Miner. Eng. 2016;98:30–39. doi: 10.1016/j.mineng.2016.07.010. [DOI] [Google Scholar]
- Lajoie-Leroux F., Dessemond C., Soucy G., Laroche N., Magnan J.-F.. Impact of the impurities on lithium extraction from β-spodumene in the sulfuric acid process. Miner. Eng. 2018;129:1–8. doi: 10.1016/j.mineng.2018.09.011. [DOI] [Google Scholar]
- Kotsupalo N. P., Menzheres L. T., Ryabtsev A. D., Boldyrev V. V.. Mechanical activation of α-spodumene for further processing into lithium compounds. Theor. Found. Chem. Eng. 2010;44(4):503–507. doi: 10.1134/S0040579510040251. [DOI] [Google Scholar]
- Gasafi E., Pardemann R.. Processing of spodumene concentrates in fluidized-bed systems. Miner. Eng. 2020;148:106205. doi: 10.1016/j.mineng.2020.106205. [DOI] [Google Scholar]
- Salakjani N. K., Singh P., Nikoloski A. N.. Acid roasting of spodumene: Microwave vs. conventional heating. Miner. Eng. 2019;138:161–167. doi: 10.1016/j.mineng.2019.05.003. [DOI] [Google Scholar]
- Zhang H., Han Y., Lai J., Wolf J., Lei Z., Yang Y., Shi F.. Direct extraction of lithium from ores by electrochemical leaching. Nat. Commun. 2024;15(1):5066. doi: 10.1038/s41467-024-48867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J. W., Kang D. J., Tran K. T., Kim M. J., Lim T., Tran T.. Recovery of lithium from Uyuni salar brine. Hydrometallurgy. 2012;117–118:64–70. doi: 10.1016/j.hydromet.2012.02.008. [DOI] [Google Scholar]
- Chung S. Y., Bloking J. T., Chiang Y. M.. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002;1(2):123–8. doi: 10.1038/nmat732. [DOI] [PubMed] [Google Scholar]
- Mu L., Zhang R., Kan W. H., Zhang Y., Li L., Kuai C., Zydlewski B., Rahman M. M., Sun C.-J., Sainio S., Avdeev M., Nordlund D., Xin H. L., Lin F.. Dopant Distribution in Co-Free High-Energy Layered Cathode Materials. Chem. Mater. 2019;31(23):9769–9776. doi: 10.1021/acs.chemmater.9b03603. [DOI] [Google Scholar]
- Li H., Li J., Zaker N., Zhang N., Botton G. A., Dahn J. R.. Synthesis of Single Crystal LiNi0.88Co0.09Al0.03O2 with a Two-Step Lithiation Method. J. Electrochem. Soc. 2019;166(10):A1956–A1963. doi: 10.1149/2.0681910jes. [DOI] [Google Scholar]
- Jugović D., Uskoković D.. A review of recent developments in the synthesis procedures of lithium iron phosphate powders. J. Power Sources. 2009;190(2):538–544. doi: 10.1016/j.jpowsour.2009.01.074. [DOI] [Google Scholar]
- Yu, W. Manufacturing method of high-property lithium salt composite-phase lithium iron phosphate material. Chinese Patent CN103531792A, 2014.
- Wang L., Qiu J., Wang X., Chen L., Cao G., Wang J., Zhang H., He X.. Insights for understanding multiscale degradation of LiFePO4 cathodes. eScience. 2022;2(2):125–137. doi: 10.1016/j.esci.2022.03.006. [DOI] [Google Scholar]
- Krause L. J., Lamanna W., Summerfield J., Engle M., Korba G., Loch R., Atanasoski R.. Corrosion of aluminum at high voltages in non-aqueous electrolytes containing perfluoroalkylsulfonyl imides; new lithium salts for lithium-ion cells. J. Power Sources. 1997;68(2):320–325. doi: 10.1016/S0378-7753(97)02517-2. [DOI] [Google Scholar]
- Abouimrane A., Ding J., Davidson I. J.. Liquid electrolyte based on lithium bis-fluorosulfonyl imide salt: Aluminum corrosion studies and lithium ion battery investigations. J. Power Sources. 2009;189(1):693–696. doi: 10.1016/j.jpowsour.2008.08.077. [DOI] [Google Scholar]
- Han H.-B., Zhou S.-S., Zhang D.-J., Feng S.-W., Li L.-F., Liu K., Feng W.-F., Nie J., Li H., Huang X.-J.. Lithium bis(fluorosulfonyl)imide (LiFSI) as conducting salt for nonaqueous liquid electrolytes for lithium-ion batteries: Physicochemical and electrochemical properties. J. Power Sources. 2011;196(7):3623–3632. doi: 10.1016/j.jpowsour.2010.12.040. [DOI] [Google Scholar]
- Yamada Y., Chiang C. H., Sodeyama K., Wang J., Tateyama Y., Yamada A.. Corrosion Prevention Mechanism of Aluminum Metal in Superconcentrated Electrolytes. ChemElectroChem. 2015;2(11):1687–1694. doi: 10.1002/celc.201500235. [DOI] [Google Scholar]
- Yamada Y., Furukawa K., Sodeyama K., Kikuchi K., Yaegashi M., Tateyama Y., Yamada A.. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J. Am. Chem. Soc. 2014;136(13):5039–5046. doi: 10.1021/ja412807w. [DOI] [PubMed] [Google Scholar]
- Na, D.-C. ; Woo, B.-W. ; Park, S.-H. ; Lee, J.-H. . Manufacturing method for lithium hexafluoro phosphate. U.S. Patent 6387340B1, 2002.
- Susarla N., Ahmed S.. Estimating Cost and Energy Demand in Producing Lithium Hexafluorophosphate for Li-Ion Battery Electrolyte. Ind. Eng. Chem. Res. 2019;58(9):3754–3766. doi: 10.1021/acs.iecr.8b03752. [DOI] [Google Scholar]
- Cai Y., Zhang H., Cao Y., Wang Q., Cao B., Zhou Z., Lv F., Song W., Duo D., Yu L.. Synthesis, application and industrialization of LiFSI: A review and perspective. J. Power Sources. 2022;535:231481. doi: 10.1016/j.jpowsour.2022.231481. [DOI] [Google Scholar]
- He, L. ; Yang, D. ; Lin, S. ; Liu, J. ; Sun, Y. . Preparation method of imidodisulfuryl fluoride lithium salt. Chinese Patent CN106044728B, 2018.
- Lim, K. Novel method for preparing lithium bis(fluorosulfonyl)imide. South Korean Patent KR101718292B1, 2017.
- Chen, Q. ; Shi, Q. ; Zheng, Z. ; Qin, J. . Preparation methods of bis(fluorosulfonyl)imide and alkali metal salts thereof. Chinese Patent CN103935970A, 2014.
- Kim J., Kim H., Ryu J. H., Oh S. M.. CommunicationLithium Bis(fluorosulfonyl)imide (LiFSI) as a Promising Salt to Suppress Solid Electrolyte Interphase Degradation at Elevated Temperatures. J. Electrochem. Soc. 2020;167(8):080529. doi: 10.1149/1945-7111/ab8fd5. [DOI] [Google Scholar]
- Holoubek J., Liu H. D., Wu Z. H., Yin Y. J., Xing X., Cai G. R., Yu S. C., Zhou H. Y., Pascal T. A., Chen Z., Liu P.. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy. 2021;6(3):303–313. doi: 10.1038/s41560-021-00783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J., Adams B. D., Zheng J., Xu W., Henderson W. A., Wang J., Bowden M. E., Xu S., Hu J., Zhang J. G.. Anode-Free Rechargeable Lithium Metal Batteries. Adv. Funct. Mater. 2016;26(39):7094–7102. doi: 10.1002/adfm.201602353. [DOI] [Google Scholar]
- Jin Y., Le P. M. L., Gao P., Xu Y., Xiao B., Engelhard M. H., Cao X., Vo T. D., Hu J., Zhong L., Matthews B. E., Yi R., Wang C., Li X., Liu J., Zhang J.-G.. Low-solvation electrolytes for high-voltage sodium-ion batteries. Nat. Energy. 2022;7(8):718–725. doi: 10.1038/s41560-022-01055-0. [DOI] [Google Scholar]
- Chen S., Zheng J., Mei D., Han K. S., Engelhard M. H., Zhao W., Xu W., Liu J., Zhang J. G.. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 2018;30(21):1706102. doi: 10.1002/adma.201706102. [DOI] [PubMed] [Google Scholar]
- Cao X., Jia H., Xu W., Zhang J.-G.. Reviewlocalized high-concentration electrolytes for lithium batteries. J. Electrochem. Soc. 2021;168(1):010522. doi: 10.1149/1945-7111/abd60e. [DOI] [Google Scholar]
- Ren X., Zou L., Cao X., Engelhard M. H., Liu W., Burton S. D., Lee H., Niu C., Matthews B. E., Zhu Z., Wang C., Arey B. W., Xiao J., Liu J., Zhang J.-G., Xu W.. Enabling high-voltage lithium-metal batteries under practical conditions. Joule. 2019;3(7):1662–1676. doi: 10.1016/j.joule.2019.05.006. [DOI] [Google Scholar]
- Kim J.-M., Yi R., Cao X., Xu Y., Engelhard M., Tripathi S., Wang C., Zhang J.-G.. Extending Calendar Life of Si-Based Lithium-Ion Batteries by a Localized High Concentration Electrolyte. ACS Energy Lett. 2024;9(5):2318–2325. doi: 10.1021/acsenergylett.4c00348. [DOI] [Google Scholar]
- Zhang X., Zou L., Xu Y., Cao X., Engelhard M. H., Matthews B. E., Zhong L., Wu H., Jia H., Ren X., Gao P., Chen Z., Qin Y., Kompella C., Arey B. W., Li J., Wang D., Wang C., Zhang J.-G., Xu W.. Advanced Electrolytes for Fast-Charging High-Voltage Lithium-Ion Batteries in Wide-Temperature Range. Adv. Energy Mater. 2020;10(22):2000368. doi: 10.1002/aenm.202000368. [DOI] [Google Scholar]
- Zheng L.-Q., Li S.-J., Lin H.-J., Miao Y.-Y., Zhu L., Zhang Z.-J.. Effects of water contamination on the electrical properties of 18650 lithium-ion batteries. Russ. J. Electrochem. 2014;50(9):904–907. doi: 10.1134/S1023193514090122. [DOI] [Google Scholar]
- Liu M., Vatamanu J., Chen X., Xing L., Xu K., Li W.. Hydrolysis of LiPF6-Containing Electrolyte at High Voltage. ACS Energy Lett. 2021;6(6):2096–2102. doi: 10.1021/acsenergylett.1c00707. [DOI] [Google Scholar]
- Byun S., Park J., Appiah W. A., Ryou M.-H., Lee Y. M.. The effects of humidity on the self-discharge properties of Li(Ni1/3Co1/3Mn1/3)O2/graphite and LiCoO2/graphite lithium-ion batteries during storage. RSC Adv. 2017;7(18):10915–10921. doi: 10.1039/C6RA28516C. [DOI] [Google Scholar]
- Fink K. E., Polzin B. J., Vaughey J. T., Major J. J., Dunlop A. R., Trask S. E., Jeka G. T., Spangenberger J. S., Keyser M. A.. Influence of metallic contaminants on the electrochemical and thermal behavior of Li-ion electrodes. J. Power Sources. 2022;518:230760. doi: 10.1016/j.jpowsour.2021.230760. [DOI] [Google Scholar]
- Zhang J.-G., Xu W., Xiao J., Cao X., Liu J.. Lithium metal anodes with nonaqueous electrolytes. Chem. Rev. 2020;120(24):13312–13348. doi: 10.1021/acs.chemrev.0c00275. [DOI] [PubMed] [Google Scholar]
- Kanamura K., Shiraishi S., Takehara Z.. Electrochemical deposition of very smooth lithium using nonaqueous electrolytes containing HF. J. Electrochem. Soc. 1996;143(7):2187–2197. doi: 10.1149/1.1836979. [DOI] [Google Scholar]
- National Chemical Standardization Technical Committee of Inorganic Chemical Sub - Technical Committee, Vol. HG/T 4066-2015. Ministry of Industry and Information Technology, China, 2015. [Google Scholar]
- National Chemical Standardization Technical Committee of Organic Chemical Sub - Technical Committee, Vol. HG/T 5391-2018. Ministry of Industry and Information Technology, China, 2018. [Google Scholar]
- National Chemical Standardization Technical Committee of Organic Chemical Sub - Technical Committee, Vol. GB/T 33107-2016. The Standardization Administration of the People’s Republic of China, China, 2016. [Google Scholar]
- National Chemical Standardization Technical Committee of Organic Chemical Sub - Technical Committee, Vol. HG/T 4790-2014. Ministry of Industry and Information Technology, China, 2014. [Google Scholar]
- Cao X.. Important factors for the reliable and reproducible preparation of non-aqueous electrolyte solutions for lithium batteries. Commun. Mater. 2023;4(1):10. doi: 10.1038/s43246-023-00338-7. [DOI] [Google Scholar]
- Lain M. J.. Recycling of lithium ion cells and batteries. J. Power Sources. 2001;97–98:736–738. doi: 10.1016/S0378-7753(01)00600-0. [DOI] [Google Scholar]
- Espinosa D. C. R., Bernardes A. M., Tenório J. A. S.. An overview on the current processes for the recycling of batteries. J. Power Sources. 2004;135(1–2):311–319. doi: 10.1016/j.jpowsour.2004.03.083. [DOI] [Google Scholar]
- Xu J., Thomas H. R., Francis R. W., Lum K. R., Wang J., Liang B.. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources. 2008;177(2):512–527. doi: 10.1016/j.jpowsour.2007.11.074. [DOI] [Google Scholar]
- Mao Z., Song Y., Zhen A. G., Sun W.. Recycling of electrolyte from spent lithium-ion batteries. Next Sustainability. 2024;3:100015. doi: 10.1016/j.nxsust.2023.100015. [DOI] [Google Scholar]
- Zachmann N., Petranikova M., Ebin B.. Electrolyte recovery from spent Lithium-Ion batteries using a low temperature thermal treatment process. J. Ind. Eng. Chem. 2023;118:351–361. doi: 10.1016/j.jiec.2022.11.020. [DOI] [Google Scholar]
- Niu B., Xu Z., Xiao J., Qin Y.. Recycling Hazardous and Valuable Electrolyte in Spent Lithium-Ion Batteries: Urgency, Progress, Challenge, and Viable Approach. Chem. Rev. 2023;123(13):8718–8735. doi: 10.1021/acs.chemrev.3c00174. [DOI] [PubMed] [Google Scholar]
- Zhang R., Shi X., Esan O. C., An L.. Organic Electrolytes Recycling From Spent Lithium-Ion Batteries. Glob. Chall. 2022;6(12):2200050. doi: 10.1002/gch2.202200050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Mu D., Zheng R., Dai C.. Supercritical CO2 extraction of organic carbonate-based electrolytes of lithium-ion batteries. RSC Adv. 2014;4(97):54525–54531. doi: 10.1039/C4RA10530C. [DOI] [Google Scholar]
- Grützke M., Mönnighoff X., Horsthemke F., Kraft V., Winter M., Nowak S.. Extraction of lithium-ion battery electrolytes with liquid and supercritical carbon dioxide and additional solvents. RSC Adv. 2015;5(54):43209–43217. doi: 10.1039/C5RA04451K. [DOI] [Google Scholar]
- Rothermel S., Evertz M., Kasnatscheew J., Qi X., Grutzke M., Winter M., Nowak S.. Graphite Recycling from Spent Lithium-Ion Batteries. ChemSusChem. 2016;9(24):3473–3484. doi: 10.1002/cssc.201601062. [DOI] [PubMed] [Google Scholar]
- Sloop, S. E. System and method for removing an electrolyte from an energy storage and/or conversion device using a supercritical fluid. U.S. Patent 7198865, 2007.
- Mouette D., Machado P. G., Fraga D., Peyerl D., Borges R. R., Brito T. L. F., Shimomaebara L. A., Dos Santos E. M.. Costs and emissions assessment of a Blue Corridor in a Brazilian reality: The use of liquefied natural gas in the transport sector. Sci. Total Environ. 2019;668:1104–1116. doi: 10.1016/j.scitotenv.2019.02.255. [DOI] [PubMed] [Google Scholar]
- Castelvecchi D.. Electric Cars: The Battery Challenge. Nature. 2021;596(7872):336–339. doi: 10.1038/d41586-021-02222-1. [DOI] [PubMed] [Google Scholar]
- Padhi A. K., Nanjundaswamy K. S., Goodenough J. B.. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997;144(4):1188–1194. doi: 10.1149/1.1837571. [DOI] [Google Scholar]
- Wang Y., Wang J., Yang J., Nuli Y.. High-Rate LiFePO4 Electrode Material Synthesized by a Novel Route from FePO4 · 4H2O. Adv. Funct. Mater. 2006;16(16):2135–2140. doi: 10.1002/adfm.200600442. [DOI] [Google Scholar]
- Kang H.-C., Jun D.-K., Jin B., Jin E. M., Park K.-H., Gu H.-B., Kim K.-W.. Optimized solid-state synthesis of LiFePO4 cathode materials using ball-milling. J. Power Sources. 2008;179(1):340–346. doi: 10.1016/j.jpowsour.2007.12.093. [DOI] [Google Scholar]
- Cheng L., Liang G., El Khakani S., MacNeil D. D.. Low cost synthesis of LiFePO4/C cathode materials with Fe2O3 . J. Power Sources. 2013;242:656–661. doi: 10.1016/j.jpowsour.2013.05.096. [DOI] [Google Scholar]
- Kilin V. I., Yakubailik E. K., Kostenenko L. P., Ganzhenko I. M.. Dressability of abagas hematite-magnetite ores. J. Min. Sci. 2012;48(2):363–368. doi: 10.1134/S1062739148020189. [DOI] [Google Scholar]
- Vincze L., Kraut B., Papp S.. A proposal for the mechanism of the photooxidation of kinetically labile transition metal complexes.: Interpretation of photoprocesses of FeSO4, FeClO4, FeCl2 in aqueous acidic solutions. Inorg. Chim. Acta. 1984;85:89–96. doi: 10.1016/S0020-1693(00)81032-4. [DOI] [Google Scholar]
- Yang S. F., Zavalij P. Y., Whittingham M. S.. Hydrothermal synthesis of lithium iron phosphate cathodes. Electrochem. Commun. 2001;3(9):505–508. doi: 10.1016/S1388-2481(01)00200-4. [DOI] [Google Scholar]
- Bouargane B., Laaboubi K., Biyoune M. G., Bakiz B., Atbir A.. Effective and innovative procedures to use phosphogypsum waste in different application domains: review of the environmental, economic challenges and life cycle assessment. J. Mater. Cycles Waste Manage. 2023;25(3):1288–1308. doi: 10.1007/s10163-023-01617-8. [DOI] [Google Scholar]
- Lv, X. ; Zhao, Z. ; Chen, L. ; Fan, L. , Method of preparing battery-grade lithium dihydrogen phosphate by recrystallized lithium hydroxide. Chinese Patent CN103531792A, 2012.
- Chen, S. , Production method of lithium dihydrogen phosphate. Chinese Patent CN101767782A, 2010.
- Yang L., Deng W., Xu W., Tian Y., Wang A., Wang B., Zou G., Hou H., Deng W., Ji X.. Olivine LiMnxFe1‑xPO4 cathode materials for lithium ion batteries: restricted factors of rate performances. J. Mater. Chem. A. 2021;9(25):14214–14232. doi: 10.1039/D1TA01526E. [DOI] [Google Scholar]
- Sun K., Luo S. H., Du N., Wei Y., Yan S.. Research progress of lithium manganese iron phosphate cathode materials: From preparation to modification. Electroanalysis. 2024;36(8):e202400120. doi: 10.1002/elan.202400120. [DOI] [Google Scholar]
- Nadoll P., Angerer T., Mauk J. L., French D., Walshe J.. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014;61:1–32. doi: 10.1016/j.oregeorev.2013.12.013. [DOI] [Google Scholar]
- Wilhelm H. A., L’Heureux J.. Natural and synthetic graphite powders: production and main industrial uses. Actual. Chim. 2006:19–22. [Google Scholar]
- Rosman A. S., Navalan R., Ramli M. M., Abd Karim N., Ahmad M. F., Johari S., Hashim N. A., Osman N. H.. Production of Low Temperature Synthetic Graphite. Int. J. Nanoelectron. Mater. 2024;16(2):469–478. doi: 10.58915/ijneam.v16i2.1254. [DOI] [Google Scholar]
- Stevensa D. A., Dahn J. R.. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000;147(4):1271–1273. doi: 10.1149/1.1393348. [DOI] [Google Scholar]
- Jensen A. B., Moseley P. L., Oprea T. I., Ellesøe S. G., Eriksson R., Schmock H., Jensen P. B., Jensen L. J., Brunak S.. Temporal disease trajectories condensed from population-wide registry data covering 6.2 million patients. Nat. Commun. 2014;5:4022. doi: 10.1038/ncomms5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. L., Yoon G., Park K. Y., Park H., Tamwattana O., Joo Kim S., Seong W. M., Kang K.. Tailoring sodium intercalation in graphite for high energy and power sodium ion batteries. Nat. Commun. 2019;10(1):2598. doi: 10.1038/s41467-019-10551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Kim Y. H., Park J. H., Susanto D., Kim J. Y., Han J., Jun S. C., Chung K. Y.. Mechanical Activation of Graphite for Na-Ion Battery Anodes: Unexpected Reversible Reaction on Solid Electrolyte Interphase via X-Ray Analysis. Adv. Sci. 2024;11(28):e2401022. doi: 10.1002/advs.202401022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, C. S. ; Amine, K. . Develop high-energy sodium-ion battery systems. Argonne National Laboratory, Jun 10–13, 2019. https://www.energy.gov/eere/vehicles/articles/develop-high-energy-sodium-ion-battery-systems. Accessed March 2, 2025. [Google Scholar]
- Cao Y., Xiao L., Sushko M. L., Wang W., Schwenzer B., Xiao J., Nie Z., Saraf L. V., Yang Z., Liu J.. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012;12(7):3783–7. doi: 10.1021/nl3016957. [DOI] [PubMed] [Google Scholar]
- Bommier C., Surta T. W., Dolgos M., Ji X.. New Mechanistic Insights on Na-Ion Storage in Nongraphitizable Carbon. Nano Lett. 2015;15(9):5888–92. doi: 10.1021/acs.nanolett.5b01969. [DOI] [PubMed] [Google Scholar]
- Stevens D. A., Dahn J. R.. The mechanisms of lithium and sodium insertion in carbon materials. J. Electrochem. Soc. 2001;148(8):A803–A811. doi: 10.1149/1.1379565. [DOI] [Google Scholar]
- Stevens D. A., Dahn J. R.. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000;147(4):1271–1273. doi: 10.1149/1.1393348. [DOI] [Google Scholar]
- Zhang B., Ghimbeu C. M., Laberty C., Vix-Guterl C., Tarascon J. M.. Correlation Between Microstructure and Na Storage Behavior in Hard Carbon. Adv. Energy Mater. 2016;6(1):1501588. doi: 10.1002/aenm.201501588. [DOI] [Google Scholar]
- Li Y., Hu Y. S., Titirici M. M., Chen L., Huang X.. Hard Carbon Microtubes Made from Renewable Cotton as High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2016;6(18):1501588. doi: 10.1002/aenm.201600659. [DOI] [Google Scholar]
- Ding J., Wang H. L., Li Z., Kohandehghan A., Cui K., Xu Z. W., Zahiri B., Tan X. H., Lotfabad E. M., Olsen B. C., Mitlin D.. Carbon Nanosheet Frameworks Derived from Peat Moss as High Performance Sodium Ion Battery Anodes. ACS Nano. 2013;7(12):11004–11015. doi: 10.1021/nn404640c. [DOI] [PubMed] [Google Scholar]
- Zhang X., Han S. C., Fan C. L., Li L. F., Zhang W. H.. Effects of pre-carbonization on the structure and performance of hard carbon in lithium cells. J. Solid State Electr. 2015;19(3):715–721. doi: 10.1007/s10008-014-2650-5. [DOI] [Google Scholar]
- Feng Y., Tao L., Zheng Z., Huang H., Lin F.. Upgrading agricultural biomass for sustainable energy storage: Bioprocessing, electrochemistry, mechanism. Energy Storage Mater. 2020;31:274–309. doi: 10.1016/j.ensm.2020.06.017. [DOI] [Google Scholar]
- Jin Q., Tao L., Feng Y., Xia D., Spiering G. A., Hu A., Moore R., Lin F., Huang H.. Agricultural Wastes for Full-Cell Sodium-Ion Batteries: Engineering Biomass Components to Maximize the Performance and Economic Prospects. ACS Sustainable Chem. Eng. 2023;11(2):536–546. doi: 10.1021/acssuschemeng.2c04750. [DOI] [Google Scholar]
- Raspado L., Speyer L., Bolmont M., Cahen S., Fontana S., Hérold C.. Tuning the porosity of hard carbons elaborated from sucrose. J. Phys. Chem. Solids. 2024;191:112013. doi: 10.1016/j.jpcs.2024.112013. [DOI] [Google Scholar]
- Kang Y. B., Weng Y. L., Han X. T., Zhang J. P., Yu X., Wang B.. Glucose-derived hard carbon/carbon nanotube neural network architectures for enhanced sodium ion storage. Carbon Lett. 2025;35:699. doi: 10.1007/s42823-024-00815-0. [DOI] [Google Scholar]
- Lu Q., Kong L. Q., Liang B., Zhao J. S.. Hard Carbon Derived from A New Type Resorcinol/2-Thenaldehyde Resin as High-Performance Anode Materials for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2022;17(12):221274. doi: 10.20964/2022.12.83. [DOI] [Google Scholar]
- Lu B., Lin C. J., Xiong H. J., Zhang C., Fang L., Sun J. Z., Hu Z. H., Wu Y. L., Fan X. H., Li G. F., Fu J. L., Deng D. R., Wu Q. H.. Hard-Carbon Negative Electrodes from Biomasses for Sodium-Ion Batteries. Molecules. 2023;28(10):4027. doi: 10.3390/molecules28104027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Yi Z., Xu R., Chen J., Cheng J., Wang Z., Liu Q., Guo Q., Xie L., Chen C.. Towards enhanced sodium storage of hard carbon anodes: Regulating the oxygen content in precursor by low-temperature hydrogen reduction. Energy Storage Mater. 2022;51:620–629. doi: 10.1016/j.ensm.2022.07.005. [DOI] [Google Scholar]
- Zhang F., Yao Y., Wan J., Henderson D., Zhang X., Hu L.. High Temperature Carbonized Grass as a High Performance Sodium Ion Battery Anode. ACS Appl. Mater. Interfaces. 2017;9(1):391–397. doi: 10.1021/acsami.6b12542. [DOI] [PubMed] [Google Scholar]
- Rudola A., Rennie A. J. R., Heap R., Meysami S. S., Lowbridge A., Mazzali F., Sayers R., Wright C. J., Barker J.. Commercialisation of high energy density sodium-ion batteries: Faradion’s journey and outlook. J. Mater. Chem. A. 2021;9(13):8279–8302. doi: 10.1039/D1TA00376C. [DOI] [Google Scholar]
- Feng Y., Tao L., He Y., Jin Q., Kuai C., Zheng Y., Li M., Hou Q., Zheng Z., Lin F., Huang H.. Chemical-enzymatic fractionation to unlock the potential of biomass-derived carbon materials for sodium ion batteries. J. Mater. Chem. A. 2019;7(47):26954–26965. doi: 10.1039/C9TA09124F. [DOI] [Google Scholar]
- Li H., Huang X. J., Chen L. Q., Wu Z. G., Liang Y.. A high capacity nano-Si composite anode material for lithium rechargeable batteries. Electrochem. Solid-State Lett. 1999;2(11):547–549. doi: 10.1149/1.1390899. [DOI] [Google Scholar]
- Xue S., Fu Y., Song Z., Chen S., Ji Y., Zhao Y., Wang H., Qian G., Yang L., Pan F.. Coil-to-Stretch Transition of Binder Chains Enabled by “Nano-Combs” to Facilitate Highly Stable SiOx Anode. Energy Environ. Mater. 2022;5(4):1310–1316. doi: 10.1002/eem2.12248. [DOI] [Google Scholar]
- Song Z., Zhang T., Wang L., Zhao Y., Li Z., Zhang M., Wang K., Xue S., Fang J., Ji Y., Pan F., Yang L.. Bio-Inspired Binder Design for a Robust Conductive Network in Silicon-Based Anodes. Small Methods. 2022;6(5):2101591. doi: 10.1002/smtd.202101591. [DOI] [PubMed] [Google Scholar]
- Huang Y. X., Ji Y. C., Zheng G. R., Cao H. B., Xue H. Y., Yao X. M., Wang L., Chen S. M., Yin Z. W., Pan F., Yang L. Y.. Tailored Interphases Construction for Enhanced Si Anode and Ni-Rich Cathode Performance in Lithium-Ion Batteries. CCS Chem. 2025;7(2):429–439. doi: 10.31635/ccschem.024.202404120. [DOI] [Google Scholar]
- Chen S., Zheng G., Yao X., Xiao J., Zhao W., Li K., Fang J., Jiang Z., Huang Y., Ji Y., Yang K., Yin Z.-W., Zhang M., Pan F., Yang L.. Constructing Matching Cathode-Anode Interphases with Improved Chemo-mechanical Stability for High-Energy Batteries. ACS Nano. 2024;18(8):6600–6611. doi: 10.1021/acsnano.3c12823. [DOI] [PubMed] [Google Scholar]
- Pinto C. R., Gamboa R. C., Henriques J. C., Serra J. M., Alves J. M., Vallera A. M.. Silicon sheet from silane: first results. Sol. Energy Mater. Sol. Cells. 2002;72(1–4):209–217. doi: 10.1016/S0927-0248(01)00166-0. [DOI] [Google Scholar]
- Zhang X., Wang D., Qiu X., Ma Y., Kong D., Mullen K., Li X., Zhi L.. Stable high-capacity and high-rate silicon-based lithium battery anodes upon two-dimensional covalent encapsulation. Nat. Commun. 2020;11(1):3826. doi: 10.1038/s41467-020-17686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li Q., Chai J., Wang Y., Du J., Chen Z., Rui Y., Jiang L., Tang B.. Si-based Anode Lithium-Ion Batteries: A Comprehensive Review of Recent Progress. ACS Mater. Lett. 2023;5(11):2948–2970. doi: 10.1021/acsmaterialslett.3c00253. [DOI] [Google Scholar]
- Qian G., Li Y., Chen H., Xie L., Liu T., Yang N., Song Y., Lin C., Cheng J., Nakashima N., Zhang M., Li Z., Zhao W., Yang X., Lin H., Lu X., Yang L., Li H., Amine K., Chen L., Pan F.. Revealing the aging process of solid electrolyte interphase on SiOx anode. Nat. Commun. 2023;14(1):6048. doi: 10.1038/s41467-023-41867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. M., Mateti S., Sultana I., Hou C., Falin A., Cizek P., Glushenkov A. M., Chen Y.. End-of-Life Photovoltaic Recycled Silicon: A Sustainable Circular Materials Source for Electronic Industries. Adv. Energy Sustainability Res. 2021;2(11):2100081. doi: 10.1002/aesr.202100081. [DOI] [Google Scholar]
- Chowdhury M. S., Rahman K. S., Chowdhury T., Nuthammachot N., Techato K., Akhtaruzzaman M., Tiong S. K., Sopian K., Amin N.. An overview of solar photovoltaic panels’ end-of-life material recycling. Energy Strateg. Rev. 2020;27:100431. doi: 10.1016/j.esr.2019.100431. [DOI] [Google Scholar]
- Preet S., Smith S. T.. A comprehensive review on the recycling technology of silicon based photovoltaic solar panels: Challenges and future outlook. J. Cleaner Prod. 2024;448:141661. doi: 10.1016/j.jclepro.2024.141661. [DOI] [Google Scholar]
- Bates J. B., Dudney N. J., Neudecker B., Ueda A., Evans C. D.. Thin-film lithium and lithium-ion batteries. Solid State Ionics. 2000;135(1):33–45. doi: 10.1016/S0167-2738(00)00327-1. [DOI] [Google Scholar]
- Zhang X., Han A., Yang Y.. Review on the production of high-purity lithium metal. J. Mater. Chem. A. 2020;8(43):22455–22466. doi: 10.1039/D0TA07611B. [DOI] [Google Scholar]
- Sato Y.. Electrowinning of Metallic Lithium from Molten Salts. ECS Proceedings Volumes. 2002;2002–19(1):771–778. doi: 10.1149/200219.0771PV. [DOI] [Google Scholar]
- Frith J. T., Lacey M. J., Ulissi U.. A non-academic perspective on the future of lithium-based batteries. Nat. Commun. 2023;14(1):420. doi: 10.1038/s41467-023-35933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, S. C. Sputtering of lithium. U.S. Patent 005830336A, 1998.
- Porthault H., Decaux C.. Electrodeposition of lithium metal thin films and its application in all-solid-state microbatteries. Electrochim. Acta. 2016;194:330–337. doi: 10.1016/j.electacta.2016.02.102. [DOI] [Google Scholar]
- Saager, S. PVD of Metallic Lithium Layers and Lithiated Silicon Layers for High-Performance Anodes in Lithium Ion Batteries. In 65th Annual Technical Conference Proceedings, 2022. 10.14332/svc22.proc.0008. [DOI] [Google Scholar]
- Rospars N., Srout M., Fu C., Mourouga G., Mensi M., Ingenito A.. High performance ultra-thin lithium metal anode enabled by vacuum thermal evaporation. Commun. Mater. 2024;5(1):179. doi: 10.1038/s43246-024-00619-9. [DOI] [Google Scholar]
- Yakovleva, M. ; Fitch, K. B. ; Xia, J. ; Greeter, W. A., Jr. . Battery utilizing printable lithium. U.S. Patent 20220149341A1, 2022.
- Xing J., Chen T., Yi L., Wang Z., Song Z., Chen X., Wei C., Zhou A., Li H., Li J.. Endowing Cu foil self-wettable in molten lithium: A roll-to-roll wet coating strategy to fabricate high-performance ultrathin lithium metal anodes. Energy Storage Mater. 2023;63:103067. doi: 10.1016/j.ensm.2023.103067. [DOI] [Google Scholar]
- Ji X., Lee K. T., Nazar L. F.. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009;8(6):500–506. doi: 10.1038/nmat2460. [DOI] [PubMed] [Google Scholar]
- Xin S., Gu L., Zhao N.-H., Yin Y.-X., Zhou L.-J., Guo Y.-G., Wan L.-J.. Smaller Sulfur Molecules Promise Better Lithium-Sulfur Batteries. J. Am. Chem. Soc. 2012;134(45):18510–18513. doi: 10.1021/ja308170k. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang J., Xie J., Xu N.. A Novel Conductive Polymer-Sulfur Composite Cathode Material for Rechargeable Lithium Batteries. Adv. Mater. 2002;14(13–14):963–965. doi: 10.1002/1521-4095(20020705)14:13/14<963::AID-ADMA963>3.0.CO;2-P. [DOI] [Google Scholar]
- Xiao L., Cao Y., Xiao J., Schwenzer B., Engelhard M. H., Saraf L. V., Nie Z., Exarhos G. J., Liu J.. A Soft Approach to Encapsulate Sulfur: Polyaniline Nanotubes for Lithium-Sulfur Batteries with Long Cycle Life. Adv. Mater. 2012;24(9):1176–1181. doi: 10.1002/adma.201103392. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhang H., Wu B., Wang M., Li X., Zhang H.. Lithium Sulfur Primary Battery with Super High Energy Density: Based on the Cauliflower-like Structured C/S Cathode. Sci. Rep. 2015;5(1):14949. doi: 10.1038/srep14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver T., Kovacik P., Marinescu M., Zhang T., Offer G.. Commercializing Lithium Sulfur Batteries: Are We Doing the Right Research? J. Electrochem. Soc. 2018;165(1):A6029–A6033. doi: 10.1149/2.0071801jes. [DOI] [Google Scholar]
- Lv D. P., Zheng J. M., Li Q. Y., Xie X., Ferrara S., Nie Z. M., Mehdi L. B., Browning N. D., Zhang J. G., Graff G. L., Liu J., Xiao J.. High Energy Density Lithium-Sulfur Batteries: Challenges of Thick Sulfur Cathodes. Adv. Energy Mater. 2015;5(16):1402290. doi: 10.1002/aenm.201402290. [DOI] [Google Scholar]
- Albertus P., Babinec S., Litzelman S., Newman A.. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy. 2018;3(1):16–21. doi: 10.1038/s41560-017-0047-2. [DOI] [Google Scholar]
- Kamaya N., Homma K., Yamakawa Y., Hirayama M., Kanno R., Yonemura M., Kamiyama T., Kato Y., Hama S., Kawamoto K., Mitsui A.. A lithium superionic conductor. Nat. Mater. 2011;10(9):682–686. doi: 10.1038/nmat3066. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Cao D., Ma Y., Natan A., Aurora P., Zhu H.. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019;31(44):1901131. doi: 10.1002/adma.201901131. [DOI] [PubMed] [Google Scholar]
- Wu B., Wang S., Evans W. J. IV, Deng D. Z., Yang J., Xiao J.. Interfacial behaviours between lithium ion conductors and electrode materials in various battery systems. J. Mater. Chem. A. 2016;4(40):15266–15280. doi: 10.1039/C6TA05439K. [DOI] [Google Scholar]
- Wang Y., Lu D., Xiao J., He Y., Harvey G. J., Wang C., Zhang J.-G., Liu J.. Superionic conduction and interfacial properties of the low temperature phase Li7P2S8Br0.5I0.5 . Energy Storage Mater. 2019;19:80–87. doi: 10.1016/j.ensm.2019.02.029. [DOI] [Google Scholar]
- Ohta N., Takada K., Sakaguchi I., Zhang L., Ma R., Fukuda K., Osada M., Sasaki T.. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 2007;9(7):1486–1490. doi: 10.1016/j.elecom.2007.02.008. [DOI] [Google Scholar]
- Kato Y., Hori S., Saito T., Suzuki K., Hirayama M., Mitsui A., Yonemura M., Iba H., Kanno R.. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy. 2016;1(4):16030. doi: 10.1038/nenergy.2016.30. [DOI] [Google Scholar]
- Lee Y.-G., Fujiki S., Jung C., Suzuki N., Yashiro N., Omoda R., Ko D.-S., Shiratsuchi T., Sugimoto T., Ryu S., Ku J. H., Watanabe T., Park Y., Aihara Y., Im D., Han I. T.. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nat. Energy. 2020;5(4):299–308. doi: 10.1038/s41560-020-0575-z. [DOI] [Google Scholar]
- Nagata H., Chikusa Y.. All-Solid-State Lithium-Sulfur Battery with High Energy and Power Densities at the Cell Level. Energy Technol. 2016;4(4):484–489. doi: 10.1002/ente.201500297. [DOI] [Google Scholar]
- Yu Z., Xu Y., Kindle M., Marty D., Deng G., Wang C., Xiao J., Liu J., Lu D.. Regenerative Solid Interfaces Enhance High-Performance All-Solid-State Lithium Batteries. ACS Nano. 2024;18(18):11955–11963. doi: 10.1021/acsnano.4c02197. [DOI] [PubMed] [Google Scholar]
- Randau S., Weber D. A., Kötz O., Koerver R., Braun P., Weber A., Ivers-Tiffée E., Adermann T., Kulisch J., Zeier W. G., Richter F. H., Janek J.. Benchmarking the performance of all-solid-state lithium batteries. Nat. Energy. 2020;5(3):259–270. doi: 10.1038/s41560-020-0565-1. [DOI] [Google Scholar]
- Albertus P., Anandan V., Ban C., Balsara N., Belharouak I., Buettner-Garrett J., Chen Z., Daniel C., Doeff M., Dudney N. J., Dunn B., Harris S. J., Herle S., Herbert E., Kalnaus S., Libera J. A., Lu D., Martin S., McCloskey B. D., McDowell M. T., Meng Y. S., Nanda J., Sakamoto J., Self E. C., Tepavcevic S., Wachsman E., Wang C., Westover A. S., Xiao J., Yersak T.. Challenges for and Pathways toward Li-Metal-Based All-Solid-State Batteries. ACS Energy Lett. 2021;6(4):1399–1404. doi: 10.1021/acsenergylett.1c00445. [DOI] [Google Scholar]
- Han F., Yue J., Zhu X., Wang C.. Suppressing Li Dendrite Formation in Li2S-P2S5 Solid Electrolyte by LiI Incorporation. Adv. Energy Mater. 2018;8(18):1703644. doi: 10.1002/aenm.201703644. [DOI] [Google Scholar]
- Janek J., Zeier W. G.. Challenges in speeding up solid-state battery development. Nat. Energy. 2023;8(3):230–240. doi: 10.1038/s41560-023-01208-9. [DOI] [Google Scholar]
- Lee K., Kim S., Park J., Park S. H., Coskun A., Jung D. S., Cho W., Choi J. W.. Selection of Binder and Solvent for Solution-Processed All-Solid-State Battery. J. Electrochem. Soc. 2017;164(9):A2075–A2081. doi: 10.1149/2.1341709jes. [DOI] [Google Scholar]
- Xiao P. T., Zhao Y., Piao Z. H., Li B. H., Zhou G. M., Cheng H. M.. A nonflammable electrolyte for ultrahigh-voltage (4.8 V-class) Li||NCM811 cells with a wide temperature range of 100 degrees C. Energy Environ. Sci. 2022;15(6):2435–2444. doi: 10.1039/D1EE02959B. [DOI] [Google Scholar]
- Sakuda A., Kuratani K., Yamamoto M., Takahashi M., Takeuchi T., Kobayashi H.. All-Solid-State Battery Electrode Sheets Prepared by a Slurry Coating Process. J. Electrochem. Soc. 2017;164(12):A2474–A2478. doi: 10.1149/2.0951712jes. [DOI] [Google Scholar]
- Lee D. J., Jang J., Lee J.-P., Wu J., Chen Y.-T., Holoubek J., Yu K., Ham S.-Y., Jeon Y., Kim T.-H., Lee J. B., Song M.-S., Meng Y. S., Chen Z.. Physio-Electrochemically Durable Dry-Processed Solid-State Electrolyte Films for All-Solid-State Batteries. Adv. Funct. Mater. 2023;33(28):2301341. doi: 10.1002/adfm.202301341. [DOI] [Google Scholar]
- Copper Mining and Processing: Processing Copper Ores. Superfund Research Center, The University of Arizona. https://superfund.arizona.edu/resources/learning-modules-english/copper-mining-and-processing/processing-copper-ores. Accessed March 2, 2025. [Google Scholar]
- Dew D. W., Phillips C. V.. The effect of Fe(ii) and Fe(iii) on the efficiency of copper electrowinning from dilute acid Cu(II) sulphate solutions with the chemelec cell. Part I. Cathodic and anodic polarisation studies. Hydrometallurgy. 1985;14:331–349. doi: 10.1016/0304-386X(85)90043-X. [DOI] [Google Scholar]
- Li, H. ; Jou, H.-J. ; Yurko, J. A. ; Kwok, W. M. R. ; Feinberg, Z. D. ; Wagman, D. C. ; Jol, E. S. ; Esmaeili, H. , Nano twin copper components. U.S. Patent 11901585B2, 2024.
- Dew D. W., Phillips C. V.. The effect of Fe(ii) and Fe(iii) on the efficiency of copper electrowinning from dilute acid Cu(II) sulphate solutions with the chemelec cell. Part II. The efficiency of copper electrowinning from dilute liquors. Hydrometallurgy. 1985;14:351–367. doi: 10.1016/0304-386X(85)90044-1. [DOI] [Google Scholar]
- Starodub K., Kuminova Y., Dinsdale A., Cheverikin V., Filichkina V., Saynazarov A., Khvan A., Kondratiev A.. Experimental Investigation and Modeling of Copper Smelting Slags. Metallurgical and Materials Transactions B. 2016;47(5):2904–2918. doi: 10.1007/s11663-016-0761-3. [DOI] [Google Scholar]
- Zhou H., Liu G., Zhang L., Zhou C.. Mineralogical and morphological factors affecting the separation of copper and arsenic in flash copper smelting slag flotation beneficiation process. Journal of Hazardous Materials. 2021;401:123293. doi: 10.1016/j.jhazmat.2020.123293. [DOI] [PubMed] [Google Scholar]
- Ye Y., Chou L.-Y., Liu Y., Wang H., Lee H. K., Huang W., Wan J., Liu K., Zhou G., Yang Y., Yang A., Xiao X., Gao X., Boyle D. T., Chen H., Zhang W., Kim S. C., Cui Y.. Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries. Nat. Energy. 2020;5(10):786–793. doi: 10.1038/s41560-020-00702-8. [DOI] [Google Scholar]
- Raabe D.. The Materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023;123(5):2436–2608. doi: 10.1021/acs.chemrev.2c00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primary Aluminium Smelting Energy Intensity. International Aluminium, 2024. https://international-aluminium.org/statistics/primary-aluminium-smelting-energy-intensity. Accessed March 2, 2025.
- Energy-Related CO2 Emission Data Tables, U.S. Energy Information Administration, Oct 29, 2024. https://www.eia.gov/environment/emissions/state/. Accessed March 2, 2025.
- Lenhart B., Zuraw M., Mustain W.. A Scalable Method for Enhancing the Crystallinity of Zn Powder to Reduce Corrosion and Boost Achievable Capacity. J. Electrochem. Soc. 2023;170:070501. doi: 10.1149/1945-7111/ace082. [DOI] [Google Scholar]
- ELYSIS progresses on the commercialization of its breakthrough technology by issuing its first smelter technology licence. Elysis, Jun 28, 2024. https://www.elysis.com/en/issuing-first-smelter-technology-licence. Accessed March 2, 2025. [Google Scholar]
- An update on our anode copper foil product & facility, Jan 19, 2022. https://www.redwoodmaterials.com/news/anode-copper-foil-product-update/. Accessed March 2, 2025.
- Lu X., Zhang Z., Hiraki T., Takeda O., Zhu H., Matsubae K., Nagasaka T.. A solid-state electrolysis process for upcycling aluminium scrap. Nature. 2022;606(7914):511–515. doi: 10.1038/s41586-022-04748-4. [DOI] [PubMed] [Google Scholar]
- Wentker M., Greenwood M., Leker J.. A Bottom-Up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials. Energies. 2019;12(3):504. doi: 10.3390/en12030504. [DOI] [Google Scholar]
- Wu B., Ouro-Koura H., Lu S.-H., Li H., Wang X., Xiao J., Deng Z. D.. Functional materials for powering and implementing next-generation miniature sensors. Mater. Today. 2023;69:333–354. doi: 10.1016/j.mattod.2023.09.001. [DOI] [Google Scholar]
- Zabara M. A., Göçmez H., Karabatak A., Ulgut B.. Characterization of Different Electrolyte Composition Lithium Thionyl Chloride Reserve Battery by Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2021;168(5):050529. doi: 10.1149/1945-7111/abff61. [DOI] [Google Scholar]
- Niu C., Liu D., Lochala J. A., Anderson C. S., Cao X., Gross M. E., Xu W., Zhang J.-G., Whittingham M. S., Xiao J., Liu J.. Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat. Energy. 2021;6(7):723–732. doi: 10.1038/s41560-021-00852-3. [DOI] [Google Scholar]
- Chen S., Niu C., Lee H., Li Q., Yu L., Xu W., Zhang J.-G., Dufek E. J., Whittingham M. S., Meng S., Xiao J., Liu J.. Critical Parameters for Evaluating Coin Cells and Pouch Cells of Rechargeable Li-Metal Batteries. Joule. 2019;3(4):1094–1105. doi: 10.1016/j.joule.2019.02.004. [DOI] [Google Scholar]
- Niu C., Lee H., Chen S., Li Q., Du J., Xu W., Zhang J.-G., Whittingham M. S., Xiao J., Liu J.. High-energy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy. 2019;4(7):551–559. doi: 10.1038/s41560-019-0390-6. [DOI] [Google Scholar]
- Liu D., Wu B., Xu Y., Ellis J., Baranovskiy A., Lu D., Lochala J., Anderson C., Baar K., Qu D., Yang J., Galvez-Aranda D., Lopez K.-J., Balbuena P. B., Seminario J. M., Liu J., Xiao J.. Controlled large-area lithium deposition to reduce swelling of high-energy lithium metal pouch cells in liquid electrolytes. Nat. Energy. 2024;9:559–569. doi: 10.1038/s41560-024-01488-9. [DOI] [Google Scholar]
- Jin S., Ye Y., Niu Y., Xu Y., Jin H., Wang J., Sun Z., Cao A., Wu X., Luo Y., Ji H., Wan L. J.. Solid-Solution-Based Metal Alloy Phase for Highly Reversible Lithium Metal Anode. J. Am. Chem. Soc. 2020;142(19):8818–8826. doi: 10.1021/jacs.0c01811. [DOI] [PubMed] [Google Scholar]
- Li-Metal completes concept study for world’s first carbonate-to-metal lithium plant, Feb 7, 2024, https://www.mining.com/li-metal-completes-concept-study-for-the-worlds-first-carbonate-to-metal-lithium-plant/. Accessed March 2, 2025.
- Li-Metal Produces Lithium Metal Ingots Using Reprocessing Technology, Nov 8, 2023. https://www.accesswire.com/800881/li-metal-produces-lithium-metal-ingots-using-reprocessing-technology. Accessed March 2, 2025.
- Nekahi A., Kumar M.R A., Li X., Deng S., Zaghib K.. Sustainable LiFePO4 and LiMnxFe1‑xPO4 (x = 0.1–1) cathode materials for lithium-ion batteries: A systematic review from mine to chassis. Materials Science and Engineering: R: Reports. 2024;159:100797. doi: 10.1016/j.mser.2024.100797. [DOI] [Google Scholar]
- Hu C., Yi H., Fang H., Yang B., Yao Y., Ma W., Dai Y.. Suppressing Li3PO4 impurity formation in LiFePO4/Fe2P by a nonstoichiometry synthesis and its effect on electrochemical properties. Mater. Lett. 2011;65(9):1323–1326. doi: 10.1016/j.matlet.2011.01.074. [DOI] [Google Scholar]
- Lu Y., Zhu T.. Status and prospects of lithium iron phosphate manufacturing in the lithium battery industry. MRS Commun. 2024;14(5):888–899. doi: 10.1557/s43579-024-00644-2. [DOI] [Google Scholar]
- He L., Xu S., Zhao Z.. Suppressing the formation of Fe2P: Thermodynamic study on the phase diagram and phase transformation for LiFePO4 synthesis. Energy. 2017;134:962–967. doi: 10.1016/j.energy.2017.06.036. [DOI] [Google Scholar]
- Kim D.-K., Park H.-M., Jung S.-J., Jeong Y. U., Lee J.-H., Kim J.-J.. Effect of synthesis conditions on the properties of LiFePO4 for secondary lithium batteries. J. Power Sources. 2006;159(1):237–240. doi: 10.1016/j.jpowsour.2006.04.086. [DOI] [Google Scholar]
- Xiao J., Adelstein N., Bi Y., Bian W., Cabana J., Cobb C. L., Cui Y., Dillon S. J., Doeff M. M., Islam S. M., Leung K., Li M., Lin F., Liu J., Luo H., Marschilok A. C., Meng Y. S., Qi Y., Sahore R., Sprenger K. G., Tenent R. C., Toney M. F., Tong W., Wan L. F., Wang C., Weitzner S. E., Wu B., Xu Y.. Assessing cathode-electrolyte interphases in batteries. Nat. Energy. 2024;9(12):1463–1473. doi: 10.1038/s41560-024-01639-y. [DOI] [Google Scholar]
- Hasan S., Islam M. S., Bashar S. M. A., Tamzid A. A. N., Hossain R. B., Haque M. A., Faishal R.. Beyond Lithium-Ion: The Promise and Pitfalls of BYD’s Blade Batteries for Electric Vehicles. E3S Web of Conferences. 2023;469:00005. doi: 10.1051/e3sconf/202346900005. [DOI] [Google Scholar]
- Colthorpe, A. , LFP cell average falls below US$100/kWh as battery pack prices drop to record low in 2023. Energy Storage News, Nov 27, 2023. https://www.energy-storage.news/lfp-cell-average-falls-below-us100-kwh-as-battery-pack-prices-drop-to-record-low-in-2023/. Accessed March 2, 2025.
- Chen Q., Lai X., Gu H., Tang X., Gao F., Han X., Zheng Y.. Investigating carbon footprint and carbon reduction potential using a cradle-to-cradle LCA approach on lithium-ion batteries for electric vehicles in China. J. Cleaner Prod. 2022;369:133342. doi: 10.1016/j.jclepro.2022.133342. [DOI] [Google Scholar]
- Deng C., Liu L., Zhou W., Sun K., Sun D.. Effect of synthesis condition on the structure and electrochemical properties of Li[Ni1/3Mn1/3Co1/3]O2 prepared by hydroxide co-precipitation method. Electrochim. Acta. 2008;53(5):2441–2447. doi: 10.1016/j.electacta.2007.10.025. [DOI] [Google Scholar]
- Wu B., Yi R., Xu Y., Gao P., Bi Y., Novák L., Liu Z., Hu E., Wang N., Rijssenbeek J., Venkatachalam S., Wu J., Liu D., Cao X., Xiao J.. Unusual Li2O sublimation promotes single-crystal growth and sintering. Nat. Energy. 2025 doi: 10.1038/s41560-025-01738-4. [DOI] [Google Scholar]
- Zheng L., Bennett J. C., Obrovac M. N.. All-Dry Synthesis of Single Crystal NMC Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2020;167(13):130536. doi: 10.1149/1945-7111/abbcb1. [DOI] [Google Scholar]
- Tahmasebi M. H., Obrovac M. N.. New Insights into the All-Dry Synthesis of NMC622 Cathodes Using a Single-Phase Rock Salt Oxide Precursor. ACS Omega. 2024;9(1):1916–1924. doi: 10.1021/acsomega.3c08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Chen X., Sushko P. V., Sushko M. L., Kovarik L., Feng J., Deng Z., Zheng J., Graff G. L., Nie Z., Choi D., Liu J., Zhang J. G., Whittingham M. S.. High-performance LiNi0.5Mn1.5O4 spinel controlled by Mn3+ concentration and site disorder. Adv. Mater. 2012;24(16):2109–16. doi: 10.1002/adma.201104767. [DOI] [PubMed] [Google Scholar]
- Schoo A., Moschner R., Hülsmann J., Kwade A.. Coating Defects of Lithium-Ion Battery Electrodes and Their Inline Detection and Tracking. Batteries. 2023;9(2):111. doi: 10.3390/batteries9020111. [DOI] [Google Scholar]
- Zheng Y., Azhari L., Meng Z., Gao G., Han Y., Yang Z., Wang Y.. The Effects of Phosphate Impurity on Recovered LiNi(0.6)Co(0.2)Mn(0.2)O(2) Cathode Material via a Hydrometallurgy Method. ACS Appl. Mater. Interfaces. 2022;14(43):48627–48635. doi: 10.1021/acsami.2c12715. [DOI] [PubMed] [Google Scholar]
- Wu B. B., Yang Y., Liu D. Y., Niu C. J., Gross M., Seymour L., Lee H., Le P. M. L., Vo T. D., Deng Z. D., Dufek E. J., Whittingham M. S., Liu J., Xiao J.. Good Practices for Rechargeable Lithium Metal Batteries. J. Electrochem. Soc. 2019;166(16):A4141–A4149. doi: 10.1149/2.0691916jes. [DOI] [Google Scholar]
- Joshi T., Eom K., Yushin G., Fuller T. F.. Effects of Dissolved Transition Metals on the Electrochemical Performance and SEI Growth in Lithium-Ion Batteries. J. Electrochem. Soc. 2014;161(12):A1915–A1921. doi: 10.1149/2.0861412jes. [DOI] [Google Scholar]
- Yang L., Chen H. S., Song W. L., Fang D.. Effect of Defects on Diffusion Behaviors of Lithium-Ion Battery Electrodes: In Situ Optical Observation and Simulation. ACS Appl. Mater. Interfaces. 2018;10(50):43623–43630. doi: 10.1021/acsami.8b15260. [DOI] [PubMed] [Google Scholar]
- Hollricher K., Strom D., Schmidt U.. Looking into batteries with Raman. Correlative RISE microscopy studies of Li-ion cells. Imaging Microsc. 2021;23:14–15. [Google Scholar]
- Patel D., Robinson J. B., Ball S., Brett D. J. L., Shearing P. R.. Thermal Runaway of a Li-Ion Battery Studied by Combined ARC and Multi-Length Scale X-ray CT. J. Electrochem. Soc. 2020;167(9):090511. doi: 10.1149/1945-7111/ab7fb6. [DOI] [Google Scholar]
- Grant P. S., Greenwood D., Pardikar K., Smith R., Entwistle T., Middlemiss L. A., Murray G., Cussen S. A., Lain M. J., Capener M. J., Copley M., Reynolds C. D., Hare S. D., Simmons M. J. H., Kendrick E., Zankowski S. P., Wheeler S., Zhu P., Slater P. R., Zhang Y. S., Morrison A. R. T., Dawson W., Li J., Shearing P. R., Brett D. J. L., Matthews G., Ge R., Drummond R., Tredenick E. C., Cheng C., Duncan S. R., Boyce A. M., Faraji-Niri M., Marco J., Roman-Ramirez L. A., Harper C., Blackmore P., Shelley T., Mohsseni A., Cumming D. J.. Roadmap on Li-ion battery manufacturing research. Journal of Physics: Energy. 2022;4(4):042006. doi: 10.1088/2515-7655/ac8e30. [DOI] [Google Scholar]
- Breaking Traditional Barriers - Collapsing Days to Seconds. https://www.6kinc.com/6k-inc-unimelt-metal-powders/unimelt-microwave-based-plasma-technology/. Accessed March 2, 2025.
- Advanced automotive battery conference, 2023, https://www.advancedautobat.com/us/. Accessed March 2, 2025.
- SMM Spot Metal Prices, 2024, https://www.metal.com. Accessed March 2, 2025.
- Schnell J., Günther T., Knoche T., Vieider C., Köhler L., Just A., Keller M., Passerini S., Reinhart G.. All-solid-state lithium-ion and lithium metal batteries - paving the way to large-scale production. J. Power Sources. 2018;382:160–175. doi: 10.1016/j.jpowsour.2018.02.062. [DOI] [Google Scholar]
- Zaman W., Hatzell K. B.. Processing and manufacturing of next generation lithium-based all solid-state batteries. Curr. Opin. Solid State Mater. Sci. 2022;26(4):101003. doi: 10.1016/j.cossms.2022.101003. [DOI] [Google Scholar]
- Balaish M., Gonzalez-Rosillo J. C., Kim K. J., Zhu Y., Hood Z. D., Rupp J. L. M.. Processing thin but robust electrolytes for solid-state batteries. Nat. Energy. 2021;6(3):227–239. doi: 10.1038/s41560-020-00759-5. [DOI] [Google Scholar]
- ENERGY.GOV. $33 Million in Funding Available To Advance Smart Manufacturing Technologies To Help Accelerate a Clean Energy Economy. Advanced Materials & Manufacturing Technologies Office, U.S. DOE, Jul 18, 2024. https://www.energy.gov/eere/ammto/articles/33-million-funding-available-advance-smart-manufacturing-technologies-help. Accessed March 2, 2025.
- Hu J., Wu B., Chae S., Lochala J., Bi Y., Xiao J.. Achieving highly reproducible results in graphite-based Li-ion full coin cells. Joule. 2021;5(5):1011–1015. doi: 10.1016/j.joule.2021.03.016. [DOI] [Google Scholar]
- Chen S., Zheng J., Yu L., Ren X., Engelhard M. H., Niu C., Lee H., Xu W., Xiao J., Liu J., Zhang J.-G.. High-efficiency lithium metal batteries with fire-retardant electrolytes. Joule. 2018;2(8):1548–1558. doi: 10.1016/j.joule.2018.05.002. [DOI] [Google Scholar]
- Li J., Fleetwood J., Hawley W. B., Kays W.. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022;122(1):903–956. doi: 10.1021/acs.chemrev.1c00565. [DOI] [PubMed] [Google Scholar]
- Attia P. M., Moch E., Herring P. K.. Challenges and opportunities for high-quality battery production at scale. Nat. Commun. 2025;16(1):611. doi: 10.1038/s41467-025-55861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang R., Wang J., Wang Y.. Current and future lithium-ion battery manufacturing. iScience. 2021;24(4):102332. doi: 10.1016/j.isci.2021.102332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battery Manufacturing - NMP Vapor & Solvent Recovery. Martek, 2025. https://www.maratek.com/battery-manufacturing. Accessed March 17, 2025. [Google Scholar]
- Kumano N., Yamaguchi Y., Akimoto Y., Ohshima A., Nakamura H., Yamamura M.. Migration of binder and conductive agent during drying process of Li-ion battery cathodes. J. Power Sources. 2024;591:233883. doi: 10.1016/j.jpowsour.2023.233883. [DOI] [Google Scholar]
- Lu Y., Zhao C.-Z., Yuan H., Hu J.-K., Huang J.-Q., Zhang Q.. Dry electrode technology, the rising star in solid-state battery industrialization. Matter. 2022;5(3):876–898. doi: 10.1016/j.matt.2022.01.011. [DOI] [Google Scholar]
- Jin W., Song G., Yoo J. K., Jung S. K., Kim T. H., Kim J.. Advancements in Dry Electrode Technologies: Towards Sustainable and Efficient Battery Manufacturing. ChemElectroChem. 2024;11(17):e202400288. doi: 10.1002/celc.202400288. [DOI] [Google Scholar]
- Gervillié-Mouravieff C., Bao W., Steingart D. A., Meng Y. S.. Non-destructive characterization techniques for battery performance and life-cycle assessment. Nat. Rev. Electr. Eng. 2024;1(8):547–558. doi: 10.1038/s44287-024-00069-y. [DOI] [Google Scholar]
- Liu, M. ; Hao, W. . Automated impurity analysis for lithium-ion batteries with Perception Software. Thermo Scientific, 2024. https://assets.thermofisher.com/TFS-Assets/MSD/Methods-&-Protocols/battery-perception.pdf. Accessed March 2, 2025.
- Zanotto F. M., Dominguez D. Z., Ayerbe E., Boyano I., Burmeister C., Duquesnoy M., Eisentraeger M., Montaño J. F., Gallo-Bueno A., Gold L., Hall F., Kaden N., Muerkens B., Otaegui L., Reynier Y., Stier S., Thomitzek M., Turetskyy A., Vallin N., Wessel J., Xu X., Abbasov J., Franco A. A.. Data Specifications for Battery Manufacturing Digitalization: Current Status, Challenges, and Opportunities. Batteries Supercaps. 2022;5(9):e202200224. doi: 10.1002/batt.202200224. [DOI] [Google Scholar]
- Ayerbe E., Berecibar M., Clark S., Franco A. A., Ruhland J.. Digitalization of Battery Manufacturing: Current Status, Challenges, and Opportunities. Adv. Energy Mater. 2022;12(17):2102696. doi: 10.1002/aenm.202102696. [DOI] [Google Scholar]
- Wunderlich, P. ; Ehteshami-Flammer, N. ; Krauß, J. ; Fitzner, A. ; Mohring, L. ; Dahmen, C. . The Power of Digitalization in Battery Cell Manufacturing. Accenture & Fraunhofer FFB Whitepaper, January 2024. https://publica.fraunhofer.de/entities/publication/8e0f75c8-04d3-4f9a-90d7-3fad51a6310c. Accessed March 2, 2025. [Google Scholar]
- Northvolt’s embrace of machine learning. Northvolt, November 2023. https://northvolt.com/articles/northvolt-machine-learning. Accessed March 2, 2025. [Google Scholar]
- Choudhary N., Clever H., Ludwigs R., Rath M., Gannouni A., Schmetz A., Hülsmann T., Sawodny J., Fischer L., Kampker A., Fleischer J., Stein H. S.. Autonomous Visual Detection of Defects from Battery Electrode Manufacturing. Adv. Intell. Syst. 2022;4(12):200142. doi: 10.1002/aisy.202200142. [DOI] [Google Scholar]
- Condon A., Buscarino B., Moch E., Sehnert W. J., Miles O., Herring P. K., Attia P. M.. A dataset of over one thousand computed tomography scans of battery cells. Data Brief. 2024;55:110614. doi: 10.1016/j.dib.2024.110614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad A., Chang J.-R., Chang T.-T.. Machine Learning Algorithm for Sorting of Battery Packs at Smart Manufacturing Industries. Advanced Production and Industrial Engineering; IOS Press. 2022:19–24. doi: 10.3233/ATDE220716. [DOI] [Google Scholar]
- Attia P. M., Grover A., Jin N., Severson K. A., Markov T. M., Liao Y. H., Chen M. H., Cheong B., Perkins N., Yang Z., Herring P. K., Aykol M., Harris S. J., Braatz R. D., Ermon S., Chueh W. C.. Closed-loop optimization of fast-charging protocols for batteries with machine learning. Nature. 2020;578(7795):397–402. doi: 10.1038/s41586-020-1994-5. [DOI] [PubMed] [Google Scholar]
- Diao W., Saxena S., Pecht M.. Accelerated cycle life testing and capacity degradation modeling of LiCoO2-graphite cells. J. Power Sources. 2019;435:226830. doi: 10.1016/j.jpowsour.2019.226830. [DOI] [Google Scholar]
- Dubarry M., Howey D., Wu B.. Enabling battery digital twins at the industrial scale. Joule. 2023;7(6):1134–1144. doi: 10.1016/j.joule.2023.05.005. [DOI] [Google Scholar]
- Wu B., Widanage W. D., Yang S., Liu X.. Battery digital twins: Perspectives on the fusion of models, data and artificial intelligence for smart battery management systems. Energy and AI. 2020;1:100016. doi: 10.1016/j.egyai.2020.100016. [DOI] [Google Scholar]
- Wang W., Wang J., Tian J., Lu J., Xiong R.. Application of Digital Twin in Smart Battery Management Systems. Chin. J. Mech. Eng. 2021;34(1):57. doi: 10.1186/s10033-021-00577-0. [DOI] [Google Scholar]
- Li W., Rentemeister M., Badeda J., Jöst D., Schulte D., Sauer D. U.. Digital twin for battery systems: Cloud battery management system with online state-of-charge and state-of-health estimation. J. Energy Storage. 2020;30:101557. doi: 10.1016/j.est.2020.101557. [DOI] [Google Scholar]
- Zhang H., Ren D., Ming H., Zhang W., Cao G., Liu J., Wang L., Song J., Qiu J., Wang J., He X., Zhang H.. Digital Twin Enables Rational Design of Ultrahigh-Power Lithium-Ion Batteries. Adv. Energy Mater. 2023;13(1):2202660. doi: 10.1002/aenm.202202660. [DOI] [Google Scholar]
- Möller S., Schwab C., Seidlmayer S., Clausnitzer M., Rosen M., Hörmann J., Mann M., Cannavo A., Ceccio G., Vacik J., Mouzakka K. F., Danner T., Latz A., Gilles R., Finsterbusch M.. The Li battery digital twin - Combining 4D modelling, electro-chemistry, neutron, and ion-beam techniques. J. Power Sources. 2024;610:234681. doi: 10.1016/j.jpowsour.2024.234681. [DOI] [Google Scholar]
- Turetskyy A., Thiede S., Thomitzek M., von Drachenfels N., Pape T., Herrmann C.. Toward Data-Driven Applications in Lithium-Ion Battery Cell Manufacturing. Energy Technol. 2020;8(2):1900136. doi: 10.1002/ente.201900136. [DOI] [Google Scholar]
- Ling C.. A review of the recent progress in battery informatics. npj Comput. Mater. 2022;8(1):1–22. doi: 10.1038/s41524-022-00713-x. [DOI] [Google Scholar]
- Lombardo T., Duquesnoy M., El-Bouysidy H., Aren F., Gallo-Bueno A., Jorgensen P. B., Bhowmik A., Demortiere A., Ayerbe E., Alcaide F., Reynaud M., Carrasco J., Grimaud A., Zhang C., Vegge T., Johansson P., Franco A. A.. Artificial Intelligence Applied to Battery Research: Hype or Reality? Chem. Rev. 2022;122(12):10899–10969. doi: 10.1021/acs.chemrev.1c00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghi S., Hidalgo M. F. V., Niri M. F., Daub R., Marco J.. Machine Learning in Lithium-Ion Battery Cell Production: A Comprehensive Mapping Study. Batteries Supercaps. 2023;6(7):e202300046. doi: 10.1002/batt.202300046. [DOI] [Google Scholar]
- Sendek A. D., Ransom B., Cubuk E. D., Pellouchoud L. A., Nanda J., Reed E. J.. Machine Learning Modeling for Accelerated Battery Materials Design in the Small Data Regime. Adv. Energy Mater. 2022;12(31):2200553. doi: 10.1002/aenm.202200553. [DOI] [Google Scholar]
- Galvez-Aranda D. E., Dinh T. L., Vijay U., Zanotto F. M., Franco A. A.. Time-Dependent Deep Learning Manufacturing Process Model for Battery Electrode Microstructure Prediction. Adv. Energy Mater. 2024;14(15):2400376. doi: 10.1002/aenm.202400376. [DOI] [Google Scholar]
- Carnevali A., Palacin M. R., Grey C. P., Franco A. A.. VOLTA: A tool for battery screening bridging the gap between virtual electrode materials and practical applications. Energy Storage Mater. 2024;67:103254. doi: 10.1016/j.ensm.2024.103254. [DOI] [Google Scholar]
- Duquesnoy M., Liu C., Kumar V., Ayerbe E., Franco A. A.. Toward high-performance energy and power battery cells with machine learning-based optimization of electrode manufacturing. J. Power Sources. 2024;590:233674. doi: 10.1016/j.jpowsour.2023.233674. [DOI] [Google Scholar]
- Niri M. F., Liu K., Apachitei G., Ramirez L. R., Lain M., Widanage D., Marco J.. Machine learning for optimized and clean Li-ion battery manufacturing: Revealing the dependency between electrode and cell characteristics. J. Cleaner Prod. 2021;324:129272. doi: 10.1016/j.jclepro.2021.129272. [DOI] [Google Scholar]
- Faraji Niri M., Apachitei G., Lain M., Copley M., Marco J.. The Impact of Calendering Process Variables on the Impedance and Capacity Fade of Lithium-Ion Cells: An Explainable Machine Learning Approach. Energy Technol. 2022;10(12):2200893. doi: 10.1002/ente.202200893. [DOI] [Google Scholar]
- Severson K. A., Attia P. M., Jin N., Perkins N., Jiang B., Yang Z., Chen M. H., Aykol M., Herring P. K., Fraggedakis D., Bazant M. Z., Harris S. J., Chueh W. C., Braatz R. D.. Data-driven prediction of battery cycle life before capacity degradation. Nat. Energy. 2019;4(5):383–391. doi: 10.1038/s41560-019-0356-8. [DOI] [Google Scholar]
- Chen B.-R., Walker C. M., Kim S., Kunz M. R., Tanim T. R., Dufek E. J.. Battery aging mode identification across NMC compositions and designs using machine learning. Joule. 2022;6(12):2776–2793. doi: 10.1016/j.joule.2022.10.016. [DOI] [Google Scholar]
- Li-Batt Design App For lithium-metal pouch cell design. PNNL, https://www.pnnl.gov/technology/li-batt-design-app. Accessed March 2, 2025.
- Franco A. A., Rucci A., Brandell D., Frayret C., Gaberscek M., Jankowski P., Johansson P.. Boosting Rechargeable Batteries R&D by Multiscale Modeling: Myth or Reality? Chem. Rev. 2019;119(7):4569–4627. doi: 10.1021/acs.chemrev.8b00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri M. N., Håkansson A., Burheim O. S., Lamb J. J.. Lithium-ion battery digitalization: Combining physics-based models and machine learning. Renewable Sustainable Energy Rev. 2024;200:114577. doi: 10.1016/j.rser.2024.114577. [DOI] [Google Scholar]
- Finegan D. P., Zhu J., Feng X., Keyser M., Ulmefors M., Li W., Bazant M. Z., Cooper S. J.. The Application of Data-Driven Methods and Physics-Based Learning for Improving Battery Safety. Joule. 2021;5(2):316–329. doi: 10.1016/j.joule.2020.11.018. [DOI] [Google Scholar]
- Gao X., Liu X., He R., Wang M., Xie W., Brandon N. P., Wu B., Ling H., Yang S.. Designed high-performance lithium-ion battery electrodes using a novel hybrid model-data driven approach. Energy Storage Mater. 2021;36:435–458. doi: 10.1016/j.ensm.2021.01.007. [DOI] [Google Scholar]
- Wang F., Zhai Z., Zhao Z., Di Y., Chen X.. Physics-informed neural network for lithium-ion battery degradation stable modeling and prognosis. Nat. Commun. 2024;15(1):4332. doi: 10.1038/s41467-024-48779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen A., Lui Y. H., Shen S., Laflamme S., Hu S., Ye H., Hu C.. Integrating physics-based modeling and machine learning for degradation diagnostics of lithium-ion batteries. Energy Storage Mater. 2022;50:668–695. doi: 10.1016/j.ensm.2022.05.047. [DOI] [Google Scholar]
- Aykol M., Gopal C. B., Anapolsky A., Herring P. K., van Vlijmen B., Berliner M. D., Bazant M. Z., Braatz R. D., Chueh W. C., Storey B. D.. PerspectiveCombining Physics and Machine Learning to Predict Battery Lifetime. J. Electrochem. Soc. 2021;168(3):030525. doi: 10.1149/1945-7111/abec55. [DOI] [Google Scholar]
- Suzumura A., Ohno H., Kikkawa N., Takechi K.. Finding a novel electrolyte solution of lithium-ion batteries using an autonomous search system based on ensemble optimization. J. Power Sources. 2022;541:231698. doi: 10.1016/j.jpowsour.2022.231698. [DOI] [Google Scholar]
- Zhang B., Merker L., Sanin A., Stein H. S.. Robotic cell assembly to accelerate battery research. Digital Discovery. 2022;1(6):755–762. doi: 10.1039/D2DD00046F. [DOI] [Google Scholar]
- Faraji Niri M., Aslansefat K., Haghi S., Hashemian M., Daub R., Marco J.. A Review of the Applications of Explainable Machine Learning for Lithium-Ion Batteries: From Production to State and Performance Estimation. Energies. 2023;16(17):6360. doi: 10.3390/en16176360. [DOI] [Google Scholar]
- Lundberg, S. M. ; Lee, S.-I. , A Unified Approach to Interpreting Model Predictions. NIPS ‘17, Proceedings of the 31st Conference on Neural Information Processing Systems, Long Beach, CA, USA, 2017; pp 4768–4777. 10.5555/3295222.3295230 [DOI] [Google Scholar]
- Adam M. L., Moses O. A., Mailoa J. P., Hsieh C.-Y., Yu X.-F., Li H., Zhao H.. Navigating materials chemical space to discover new battery electrodes using machine learning. Energy Storage Mater. 2024;65:103090. doi: 10.1016/j.ensm.2023.103090. [DOI] [Google Scholar]
- Vlijmen B. v., Lam V., Asinger P. A., Cui X., Ganapathi D., Sun S., Herring P. K., Gopal C. B., Geise N., Deng H. D., Thaman H. L., Kang S. D., Trewartha A., Anapolsky A., Storey B. D., Gent W. E., Braatz R. D., Chueh W. C.. Interpretable Data-Driven Modeling Reveals Complexity of Battery Aging. ChemRxiv. 2023 doi: 10.26434/chemrxiv-2023-zdl2n-v2. [DOI] [Google Scholar]
- Friedman J. H., Popescu B. E.. Predictive learning via rule ensembles. Ann. Appl. Stat. 2008;2(3):916–954. doi: 10.1214/07-AOAS148. [DOI] [Google Scholar]
- Svensson P. H., Yushmanov P., Tot A., Kloo L., Berg E., Edström K.. Robotised screening and characterisation for accelerated discovery of novel Lithium-ion battery electrolytes: Building a platform and proof of principle studies. Chem. Eng. J. 2023;455:140955. doi: 10.1016/j.cej.2022.140955. [DOI] [Google Scholar]
- Dave A., Mitchell J., Burke S., Lin H., Whitacre J., Viswanathan V.. Autonomous optimization of non-aqueous Li-ion battery electrolytes via robotic experimentation and machine learning coupling. Nat. Commun. 2022;13(1):5454. doi: 10.1038/s41467-022-32938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil, S. ; Gaikwad, D. ; Sebastian, B. ; Dubey, A. ; Upadhyay, A. . Semi-Autonomous Robotic System for Efficient Recycling of Lithium-Ion Batteries. International Conference on E-mobility, Power Control and Smart Systems (ICEMPS); IEEE, 2024. 10.1109/ICEMPS60684.2024.10559331. [DOI] [Google Scholar]
- Harper G., Sommerville R., Kendrick E., Driscoll L., Slater P., Stolkin R., Walton A., Christensen P., Heidrich O., Lambert S., Abbott A., Ryder K., Gaines L., Anderson P.. Recycling lithium-ion batteries from electric vehicles. Nature. 2019;575(7781):75–86. doi: 10.1038/s41586-019-1682-5. [DOI] [PubMed] [Google Scholar]
- Fan E., Li L., Wang Z., Lin J., Huang Y., Yao Y., Chen R., Wu F.. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020;120(14):7020–7063. doi: 10.1021/acs.chemrev.9b00535. [DOI] [PubMed] [Google Scholar]
- Secretary Wright Acts to “Unleash Golden Era of American Energy Dominance”. U.S. Department of Energy, Feb 5, 2025. https://www.energy.gov/articles/secretary-wright-acts-unleash-golden-era-american-energy-dominance. Accessed March 17, 2025.
- Lai X., Chen Q., Tang X., Zhou Y., Gao F., Guo Y., Bhagat R., Zheng Y.. Critical review of life cycle assessment of lithium-ion batteries for electric vehicles: A lifespan perspective. eTransportation. 2022;12:100169. doi: 10.1016/j.etran.2022.100169. [DOI] [Google Scholar]
- Kallitsis E., Korre A., Kelsall G. H.. Life cycle assessment of recycling options for automotive Li-ion battery packs. J. Cleaner Prod. 2022;371:133636. doi: 10.1016/j.jclepro.2022.133636. [DOI] [Google Scholar]
- Zhang W.-J.. Structure and performance of LiFePO4 cathode materials: A review. J. Power Sources. 2011;196(6):2962–2970. doi: 10.1016/j.jpowsour.2010.11.113. [DOI] [Google Scholar]
- Geng J., Gao S., Sun X., Liu Z., Zhao F., Hao H.. Potential of electric vehicle batteries second use in energy storage systems: The case of China. Energy. 2022;253:124159. doi: 10.1016/j.energy.2022.124159. [DOI] [Google Scholar]
- Zhao Y., Kang Y., Wozny J., Lu J., Du H., Li C., Li T., Kang F., Tavajohi N., Li B.. Recycling of sodium-ion batteries. Nature Reviews Materials. 2023;8(9):623–634. doi: 10.1038/s41578-023-00574-w. [DOI] [Google Scholar]
- Ahuis M., Doose S., Vogt D., Michalowski P., Zellmer S., Kwade A.. Recycling of solid-state batteries. Nat. Energy. 2024;9(4):373–385. doi: 10.1038/s41560-024-01463-4. [DOI] [Google Scholar]
- Fan J., Luo H., Wang T., Dai S.. Progress in direct recycling of spent lithium nickel manganese cobalt oxide (NMC) cathodes. Energy Storage Mater. 2024;73:103813. doi: 10.1016/j.ensm.2024.103813. [DOI] [Google Scholar]
- Ji H., Wang J., Ma J., Cheng H. M., Zhou G.. Fundamentals, status and challenges of direct recycling technologies for lithium ion batteries. Chem. Soc. Rev. 2023;52(23):8194–8244. doi: 10.1039/D3CS00254C. [DOI] [PubMed] [Google Scholar]
- Qian G., Li Z., Wang Y., Xie X., He Y., Li J., Zhu Y., Xie S., Cheng Z., Che H., Shen Y., Chen L., Huang X., Pianetta P., Ma Z.-F., Liu Y., Li L.. Value-creating upcycling of retired electric vehicle battery cathodes. Cell Rep. Phys. Sci. 2022;3(2):100741. doi: 10.1016/j.xcrp.2022.100741. [DOI] [Google Scholar]
- Wilkinson, A. ; Rengarajan, S. , The US Needs To Produce Batteries, Not Just EVs. Industry Today, Nov 30, 2021. https://industrytoday.com/the-us-needs-to-produce-batteries-not-just-evs/. Accessed March 2, 2025.
- Battery Workforce Initiative, https://netl.doe.gov/bwi. Accessed March 2, 2025.
- Udavant, S. How the Southeast US is preparing a battery manufacturing talent pipeline. ManufacturingDive, Aug 7, 2023. https://www.manufacturingdive.com/news/battery-manufacturing-workforce-development-georgia-south-carolina-sk-on/688295/. Accessed March 2, 2025.
- Denisart L., Troncoso J. F., Loup-Escande E., Franco A. A.. Breaking Down the Barriers between the Digital and the Real: Mixed Reality Applied to Battery Manufacturing R&D and Training. Batteries Supercaps. 2024;7(7):e202400042. doi: 10.1002/batt.202480702. [DOI] [Google Scholar]















