Abstract
Background
The two subtypes of primary progressive aphasia (PPA) associated with Frontotemporal Lobar Degeneration (FTLD)—non-fluent (nfvPPA) and semantic (svPPA)—have distinct structural and functional abnormalities. Transcranial direct current stimulation (tDCS) targets the language network to address deficits, yet a single, arbitrary montage is often applied despite pathophysiological differences. Since tDCS current distribution depends on brain structure and function, variant-specific montages are essential. This study presents a pathology-specific approach for tDCS montage selection, identifying the optimal montage for each PPA variant.
Method
T1-weighted and resting-state fMRI data from 38 healthy, 31 nfvPPA and 32 svPPA subjects were obtained. Grey matter volume and functional entropy were analysed across 116 brain regions. Patients and controls were compared to identify significant differences in atrophy and entropy. Electric-field modelling of three widely used dorsal, ventral, and frontal tDCS montages provided current intensity estimates in the language network. Canonical Correlation Analysis examined the relationship between current intensity, atrophy, and entropy.
Results
Structural and functional changes differed between the two PPA variants: nfvPPA showed left frontal atrophy and reduced entropy in the left parietal/cerebellar areas, while svPPA exhibited left temporal atrophy and reduced entropy in the left frontal and right temporal regions. Atrophy distribution primarily influenced tDCS current spread, determining montage suitability. In nfvPPA, the frontal montage showed a strong association between delivered current and grey mettwr volume of atrophied areas, whereas in svPPA, a similar pattern was observed for the ventral montage.
Conclusion
The study identifies the frontal montage as the most suitable for nfvPPA and the ventral montage for svPPA. This study highlights the importance of pathology-specific tDCS montage selection, emphasizing the need for variant-based modulation of the language network in PPA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-025-01737-3.
Keywords: Pathology; Specific tDCS, Primary progressive aphasia; Semantic and non; Fluent, structural atrophy, functional entropy, current; Atrophy; Entropy relationship
Introduction
Language impairment caused by neurodegenerative processes characterises the two distinct variants of primary progressive aphasia (PPA)- non-fluent (nfvPPA) and semantic (svPPA) [1, 2]. The nfvPPA variant involves slow, effortful speech with grammatical errors and difficulty in understanding complex instructions [1]. The svPPA variant is marked by naming difficulties, impaired single-word comprehension, surface dyslexia or dysgraphia, and reduced object knowledge [1, 2]. Both variants affect the left hemisphere dominant language network in the brain, though each disorder impairs distinct cortical regions [3]. Due to pharmacological limitations in the treatment of PPA, the use of transcranial-direct-current-stimulation (tDCS) as a rehabilitative approach has gained momentum [4]. The current injected by tDCS montage (electrodes positioned on the scalp) is distributed across the cortical areas depending on the structural and function organisation of the brain [5–7]. Since the organisation differs in nfvPPA and svPPA, investigating pathology-specific montages that can target the language network underlying each variant is essential.
In nfvPPA, the left inferior frontal gyus (IFG) is the main area of cortical atrophy that spreads to parietal areas of the dorsal language network including the left inferior parietal lobule (IPL) and supramarginal gyrus (SMG) [8]. In svPPA, the main atrophy hub is the left anterior lobe (ATL) and middle temporal gyrus (MTG), that connect to the IFG as part of the ventral language stream [8]. Very few studies have investigated the effect of tDCS stimulation on the atrophied brain regions in the two PPA variants. A study with a larger cohort (n = 36) investigated the effects of stimulating the left IFG (language hub) on the task performed by PPA patients across both variants [9]. Significant behavioural effects were found in trained items that generalised to untrained items following tDCS for nfvPPA, but only in trained items for svPPA [9]. Same experimental protocol but variant-based differential findings highlighted how targeting the regions of atrophy, which varies by variant, influences the benefits of tDCS [10]. Similarly, using resting-state functional magnetic resonance imaging (rsfMRI), a study has shown tDCS-related behavioural improvements in PPA to correlate with changes in connectivity between the left IFG and other temporal language areas (specifically between the left IFG triangularis and the left inferior temporal gyrus (ITG), and between the left IFG opercularis and the left MTG) [11]. But the association between functional brain properties and tDCS-induced current distribution is yet to be elucidated.
Electric-field (EF) modelling of tDCS current in the brain has provided accurate estimations of the spread of current across brain regions [12, 13]. Experimental in-vivo validation of these models has facilitated their wide applicability [14, 15]. The tDCS studies have reported significant correlations between brain atrophy and estimated current densities [16–19]. In PPA, only one study has explored how distinct atrophy patterns across variants influence tDCS current flow using EF modelling [20]. They analysed single-subject brain images of different PPA variants using frontal tDCS montages targeting the left-IFG with a 5 × 5 cm2 anode and an extracephalic cathode (same as the frontal montage used here). They found no difference in current distribution according to PPA variant. This finding was natural since the study was limited to a single image per variant with moderate atrophy and with simulations performed for only one montage. The same group contradicted the finding [9], as they found significant benefits of tDCS over the left IFG for nfvPPA compared to svPPA. The discrepancy in findings appeals to the application of EF modelling on a larger cohort of nfvPPA and svPPA to identify the variant-specific tDCS montages. In this aspect, three montages (dorsal, ventral, and frontal) that can target the hubs of the language network have emerged and are widely used [9, 21]. The estimate of current-intensity received by the regions targeted by each montage can be combined with structural and functional characteristics of each brain area (affected by the disorder) to investigate how the regional attributes of structure and function influence tDCS current distribution in the language network across the PPA variants.
Two parameters that can characterise a brain region’s structural and functional attributes are atrophy and entropy. Regional atrophy, reflecting the loss of brain tissue, is a characteristic feature of PPA. Similarly brain entropy estimated from the resting-state functional MRI (rsfMRI) of a region is a measure of the variability of brain signals and an indicator of the brain’s adaptive capacity [22]. Reduced entropy, often a result of atrophy, signifies diminished complexity and variability in neural signals, leading to more predictable and less flexible neural activity patterns [23]. This relationship between atrophy, entropy, and aging has provided a foundation for studying neuropsychiatric disorders [24]. Brain entropy has been explored extensively in various research domains. For instance, it has been used to investigate states of consciousness, including during anaesthesia [25–28], altered states, and disorders of consciousness [29–34]. Additionally, individual differences in brain entropy have been linked to intelligence, cognitive abilities [35, 36], ageing, and developmental processes [37–41]. Neuromodulation techniques, such as transcranial direct current stimulation (tDCS), have shown the potential to enhance brain entropy by increasing neural activity, restoring normal neural patterns, and improving information processing [16, 42–47]. However, there is currently no information on brain entropy in patients with PPA or its potential role in language outcomes. Changes in atrophy and entropy hold significant clinical value as potential biomarkers for assessing treatment efficacy, particularly in therapeutic interventions aimed at neurological and cognitive disorders.
In the present study, we framed a multimodal approach to estimate the contribution of structural and functional parameters towards determining variant-specific tDCS montage in PPA. To this, we first identified differences in regional structural (atrophy) and functional (entropy) characteristics between each variant of PPA and healthy individuals. Second, we evaluated the current intensity at four language hubs (left-IFG-pars-opercularis, left-IFG-pars-triangularis, left-IPL, and left-MTG) by simulating three standard tDCS montages commonly used in language studies. Finally, we used canonical correlation analysis (CCA) to evaluate the relationship between multivariate sets (tDCS-current-intensity, atrophy, and entropy) and infer the suitability of each montage across the PPA variants.
Methodology
Overview
The overall approach of the study is shown in Fig. 1. In short, EF modelling of the structural images of the three groups (Healthy, nfvPPA and svPPA) was performed to estimate the current density in the target areas of the language network using three standard tDCS montages. These montages have been widely used in previous studies for stimulating the hubs of language network [9, 48–50]. This was followed by estimation of regional grey-matter atrophy from structural images and calculation of regional brain entropy from rsfMRI. The interplay between current-intensity, atrophy and entropy was analysed using CCA.
Fig. 1.
shows the (i) position of electrodes for the dorsal, ventral, and frontal montage, and (ii) the average current density (ACD) at the target ROIs for each montage. (iii) Schematic representation of the inputs and outputs of Generalized Additive Kernel Canonical Correlation Analysis (GAKCCA) used in the study to explore the suitability of the montages across the two variants of PPA
Data description
Structural (T1-weighted) and functional (rsfMRI) neuroimaging data from a total of 175 subjects across the three groups (Healthy, nfvPPA, and svPPA) were downloaded from the Frontotemporal Lobar Degeneration Neuroimaging initiative (NIFD, study details available at https://www.allftd.org/study-information). The study was coordinated through the University of California, San Francisco (UCSF), Memory and Aging Centre and these subjects were the cohort recruited across the three sites. Neurological, radiological and functional assessment categorised the patients to the respective variants of PPA [1]. To avoid site variation, we limited our subject selection in the present study to UCSF, using a 3 T Siemens Trio Tim system with a 12-channel head coil, and included only those with both structural and functional images available. The structural images were acquired using MPRAGE sequence with TR/TE = 2300/2.9, isotropic voxel = 1 mm3, with slice thickness = 1 mm. The functional images were acquired for 8 min (240 volumes) using EPI sequence with TR/TE = 2000/27, voxel = 2.5 × 2.5 × 3.6 mm3, with slice thickness = 3 mm. In the present analysis, age and sex matching identified (total N = 101) 38 healthy (age 63 ± 7, 19 Male), 31 nfvPPA (age 68 ± 6, 15 Male), and 32 svPPA (age 64 ± 7, 12 Male) subjects. The dataset did not contain data for the logopenic or mixed variant of PPA.The present study is approved by the National Institute of Mental Health and Neuro Sciences (NIMHANS) ethical review board [NIMHANS/39 th IEC (BS & NS DIV.)/].
Average Current density (ACD) at target ROI
Three montages that have been used to modulate the language network in prior research [9, 21, 51] were chosen for the simulation study: (1) Dorsal Montage- with anode at CP5 and cathode at CZ targets the left IPL in the dorsal pathway [21, 52], (2) Ventral Montage- with anode at TP7 and cathode at the nape-of-the-neck can appropriately target the left MTG/ITG in the ventral pathway [21, 52], and (3) Frontal Montage- with anode placed at F7 and cathode at extracephalic right cheek targets the left IFG, where both the dorsal and ventral montages converge [9, 49] (Fig. 1i). The montages simulated in our work are taken from previous studies that have used these montages to stimulate the underlying language network [9, 11, 49, 53–55]. For example, a prior simulation study that evaluated 10 different montage configurations across three electrode sizes found dorsal and ventral montage (used in present study) to appropriately target the language areas of each route [48]. Similarly, frontal montage (considered here) was taken from previous clinical trials that have reported the montage to be effective in rehabilitation of PPA patients [2–7]. The conventional electrode size 5 × 5 cm2 was used for simulating 113 (38 healthy, 31 nfvPPA, and 32 svPPA) tDCS procedures with a standard 2 mA current dose with three montage configurations, totalling 339 simulations in ROAST [56]. Default tissue conductivity values (white matter: 0.126 S/m, grey matter: default 0.276 S/m, cerebrospinal fluid:1.65 S/m, bone: default:0.01 S/m, skin: default 0.465 S/m, air: 2.5e−14 S/m, gel: 0.3 S/m, electrode: 5.9e7 S/m) were applied. The ROAST simulation provided coordinates and current density values for all the brain voxels. The i-SATA (MNI) tDCS simulation pipeline [13] maps the brain voxels to the Automated Anatomical Labelling (AAL- 116) atlas [57] with 116 brain regions (90 cortical and subcortical areas, and 26 cerebellar regions). For each montage, the average current density (ACD) at the 4 target ROIs: Left inferior parietal lobule (L_IPL), Left middle temporal gyrus (L_MTG), Left inferior frontal gyrus pars opercularis (L_IFG_P.Opercularis) and Left inferior frontal gyrus pars triangularis (L_IFG_P. triangularis) was calculated.
Estimation of GM volumes
The volume parameters for each T1 image were computed using the CAT12 toolbox (version 12.7) [8]. The process included correcting for bias-field inhomogeneity and spatial normalization via the DARTEL algorithm. Images were segmented into grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) volumes. To adjust for individual brain size differences, the segmented images were modulated by multiplying tissue class images aligned with the template by the Jacobian determinant from spatial normalization [8, 9]. Finally, the segmented images were smoothed using an isotropic gaussian kernel with an 8-mm FWHM [8]. This was followed by regional estimation of GM volumes using the AAL- 116 atlas [58, 59]. The GM matter volume measure was normalised for total intracranial volume for each region as .
Entropy estimation
rsfMRI preprocessing and individualised rsfMRI timeseries extraction
The rsfMRI scans were pre-processed using the standard preprocessing pipeline delineated in SPM12 software package [60]. Specifically, the images were first reoriented to the AC-PC line with the anterior commissure as the point of origin, then slice-time corrected, and realigned to adjust for head movements. The images were then co-registered to the T1-weighted image and then normalized to transform the data into standard Montreal Neurological Institute (MNI) space. Smoothing with a Gaussian kernel of 6 × 6 × 6 mm full-width at half-maximum (FWHM) was applied and the data were s band-pass filtered (0.008 < f < 0.09 Hz). White matter and CSF signals (5 regressors each), and motion parameters (6 motion parameters with their 1 st order temporal derivatives) were regressed from the rsfMRI signals using component-based noise correction method (CompCor). Finally, parcellated data was obtained using the AAL- 116 region atlas. The rsfMRI for each subject was 116 regions × 240 (no of volume per image) matrices.
An individualisation technique was applied in which rsfMRI of all subjects in a group are stacked together and the first component common across all the subjects was removed. In our previous work, we have found that individualising rsfMRI scans leads to removal of spurious environmental (scanner noise) and experimental effects that enhances the brain-behaviour association and improves the understanding of the underlying pathology [61–63].
Multiscale brain entropy estimation
Entropy measures the degree of randomness in the data and multiscale brain entropy for each individual’s rsfMRI was calculated [41, 64–66]. The entropy is the “logarithmic likelihood” that a small section (within a window of a length ‘m’) of the data that “matches” with other sections will still “match” when ‘m’ is increased by 1. Since stochastic signals do not match absolutely, a threshold ‘r’ (Tolerance factor) is defined. To account for the flexibility at multiple time scales, a scale factor ‘’ that allows coarse-graining of the signal is implemented [41, 64]. The selection of these entropy parameters (m, r, and ) is based on maximizing the difference in the entropy estimates between the two groups (healthy and patient) [16, 23, 41, 64, 67–69]. Most studies have found m to range from 1 to 3, r to range from 0.20 to 0.5, and to vary from 1 to 5. We followed a similar approach and outlined a range of parameters- m = 1 to 4, r = 0.10 to 0.60, and scale factor = 1 to 5. The parameters m and were incremented by 1, and r was incremented at the rate of 0.01.
Comparison of current, atrophy, entropy in PPA with healthy and across groups
Atrophied regions were identified as those with significant differences in normalized GM volumes between healthy individuals and both nfvPPA and svPPA groups, using a two-tailed t-test with False Discovery Rate (FDR) correction.
Similarly, The differences in entropy in each AAL region between the healthy and patient group (nfvPPA and svPPA) were estimated for each parametric value (m, r, and ). Bootstrapping was done (5000 samples with replacement) with false discovery rate (FDR) adjustments at 0.05 to observe the differences at each AAL region. The number of AAL regions with a significant difference (p < 0.05, FDR adjusted) in entropy between the two groups was counted. The entropy (m, r and ) parameters for which the count (number of areas significantly different between the groups) was maximum were finalized. For further details on entropy, refer to supplementary section 1.
The current at the target ROI was compared between healthy individuals and PPA groups, as well as between nfvPPA and svPPA. Similarly, common regions of atrophy and entropy between nfvPPA and svPPA were analysed using a two-tailed t-test with False Discovery Rate (FDR) correction. Lastly, atrophy and entropy measures were correlated with behavioural measures using Pearson correlation.
Association between tDCS current, structural atrophy and functional entropy
Generalized Additive Kernel Canonical Correlation Analysis (GAKCCA), an extension of canonical correlational analysis (CCA), handles multi-group and nonlinear relationships, providing insights into each variable’s contributions for in-depth analysis. Built on previous CCA methods, GAKCCA estimates nonlinear relationships between groups using an additive model with permutation-based tests (see supplementary section 2 for details on GAKCCA).
For each PPA group (total two groups) and tDCS montages (total three montages), GAKCCA estimates the association between the measures of regions with significant atrophy (Y1 atrophy matrix), entropy (Y2 entropy matrix), and estimation of current density as ACD at target ROIs (Y3 current matrix) as demonstrated in Fig. 1(iii). For both the groups, Y1 and Y2 matrix consisted of cortical regions whose measures (atrophy and entropy) were significantly different from healthy individuals (i.e., Number of subjects in group × Number of significant areas). Y3 consisted of ACD values at 4 target ROIs (Left_IPL, Left_MTG, Left_IFG_P.Opercularis, and Left_IFG_P. triangularis) obtained from EF modelling of dorsal, ventral, and frontal montages (totalling to 4 × 3 = 12 ACD values). For the convenience of our readers, we have named the ACD values at the target ROIs by prefixing the kind of montage (dorsal, ventral, and frontal-D, V and F) for which it was obtained. For example, ‘D_L_Inferior Parietal_Lobule’ refers to ACD values at Left_IPL obtained from EF modelling of dorsal montage, ‘V_L_Inferior Parietal_Lobule’ refers to the ACD value for ventral montage, and similarly ‘F_L_Inferior Parietal_Lobule’ to the ACD values for frontal montage.
GAKCCA employs a gaussian kernel for each variable with median based bandwidth, fully connected design matrix and a horst-scheme function to obtain the estimates of the relationships between the multiple groups. To estimate the significance (p values) of each variable we set the number of samples for the permutation test to 10,000. The correlation between the three groups () and the combination of the function of the covariances across the groups
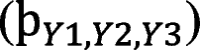 are obtained. The contribution coefficient () of the variables in the group provided the estimate of the ccontribution of one variable, say, X11 in a group X1 = (X11, …, X1p1)T in relation to the another group X2 = (X21, …, X2p2)T. To estimate the significance (p-values) of each variable, we use permutation test for null hypothesis testing, following Bae et al. (2022) [70]. This process involves calculating the observed correlation from the original data, then resampling each group with replacement, and recalculating the correlation across 10,000 iterations to form an empirical distribution. We reject the null hypothesis if the percentage of resampled correlations exceeding the observed correlation falls below a set significance level of 0.05.
are obtained. The contribution coefficient () of the variables in the group provided the estimate of the ccontribution of one variable, say, X11 in a group X1 = (X11, …, X1p1)T in relation to the another group X2 = (X21, …, X2p2)T. To estimate the significance (p-values) of each variable, we use permutation test for null hypothesis testing, following Bae et al. (2022) [70]. This process involves calculating the observed correlation from the original data, then resampling each group with replacement, and recalculating the correlation across 10,000 iterations to form an empirical distribution. We reject the null hypothesis if the percentage of resampled correlations exceeding the observed correlation falls below a set significance level of 0.05.
We further examined how the current delivered to the target areas (by each montage) associates with the areas that are- structurally atrophied and with altered entropy. To accomplish this, we evaluated the correlation between each region in the atrophy (Y1) and entropy (Y2) matrices with the ACD values of the 4 tDCS target ROIs for each dorsal, ventral, and frontal montage. The areas with atrophy and entropy values that significantly (p < 0.05) correlated with the ACDs of each montage were identified. The matrix with entries of p-values was referred to as ‘montage-significance matrix’.
Results
Patient description
The NIFD study collected a large set of behavioural measures for both nfvPPA and svPPA patients (details available in the website). We have consolidated the scores of the few language-deficit related behavioural measures in Table 1.
Table 1.
The scores (mean ± std) of various dementia and language-deficit-related behavioural measures, along with their significant differences from controls and across groups (p-values), are presented
| Behaviour | nfvPPA (mean ± std) | svPPA (mean ± std) | Healthy (mean ± std) | nfvPPA vs Healthy (t-value) | nfvPPA vs Healthy (p-value) | svPPA vs Healthy (t-value) | svPPA vs Healthy (p-value) | nfvPPA vs svPPA (t-value) | nfvPPA vs svPPA (p-value) |
|---|---|---|---|---|---|---|---|---|---|
| Clinical Dementia Rating | 0.29 ± 0.18 | 0.64 ± 0.05 | 0.03 ± 0.02 | 8.01 | 5.4e−09*** | 65.7 | 2.8e−40*** | − 10.4 | 3.2e−12*** |
| Mini Mental State Exam | 24.16 ± 0.48 | 22.7 ± 0.62 | 29.2 ± 0.11 | − 58.4 | 4.5e−34*** | − 59.07 | 1.8e−34*** | 10.1 | 1.5e−14*** |
| Boston Naming Test (15-item) | 11.94 ± 0.72 | 5.27 ± 0.69 | 14.4 ± 0.11 | − 19.04 | 1.2e−18*** | − 74.4 | 1.9e−37*** | 37.5 | 1.09e−43*** |
| Peabody Picture Vocabulary Test Verbal | 3.55 ± 0.19 | 2.67 ± 0.36 | 3.18 ± 0.45 | 5.12 | 2.4e-06*** | − 5.66 | 2.48e−07*** | 12.1 | 3.3e−16*** |
| Verbal fluency | 8.25 ± 2.19 | 7.26 ± 1.14 | 16.52 ± 1.10 | − 19.55 | 6.3e−22*** | − 36.3 | 1.09e−44*** | 2.2 | 3.0e−02*** |
| Geriatric Depression Scale | 3.69 ± 1.37 | 5.68 ± 1.87 | 1.40 ± 0.74 | 8.56 | 1.1e−10*** | 12.3 | 9.7e−15*** | − 4.8 | 1.0e−05*** |
Comparison of atrophy, entropy, and tDCS current within and across groups
Comparison of PPA with healthy
The grey-matter volume and entropy across 116 regions were calculated for the three groups. The entropy (m, r and ) parameters for (i) nfvPPA were m = 2, r = 0.35 and = 2, and (ii) svPPA were m = 2, r = 0.32 and = 2, respectively. Figure 2 displays the mean atrophy and entropy values for nfvPPA (i and iii) and svPPA (ii and iv) for areas where their values significantly (p < 0.05, FDR corrected) differed from those of healthy individuals. In nfvPPA, 33 regions showed significant (p < 0.05, FDR corrected) volume differences compared to healthy, while 20 regions had significant (p < 0.05, FDR corrected) entropy differences. Only 6 regions of left hemisphere (Rolandic operculum, amygdala, putamen, Heschl gyrus, inferior parietal lobe, and supramarginal gyrus) with significant atrophy overlapped with the regions with significant entropy differences Similarly, for svPPA, significant volume (p < 0.05, FDR corrected) differences were found in 32 regions, and 20 regions showed significant (p < 0.05, FDR corrected) entropy differences. Only 3 regions (right insula, left anterior cingulum and right amygdala) with significant atrophy overlapped with the regions with significant entropy differences The differences in current-intensity at the 4 target ROIs (compared to healthy) obtained from EF modelling of dorsal, ventral, and frontal montages (totalling to 4 × 3 = 12 ACD values) is shown for nfvPPA (Fig. 2 (v)) and svPPA (Fig. 2 (vi)) groups. It is evident that volume, entropy, and ACD at target ROIs decrease in both PPA variants compared to healthy individuals.
Fig. 2.
(i) and (ii) shows the change in the mean grey matter volume in the nfvPPA and svPPA groups that were significantly different (p < 0.05, FDR corrected) from healthy individuals. Similarly, (iii) and (iv) shows the changes in the mean values of entropy in the nfvPPA and svPPA groups that were significantly different (p < 0.05, FDR corrected) from healthy individuals. (v) and (vi) shows the changes in the mean values of ACD at the 4 target ROIs for both the nfvPPA and svPPA groups compared to healthy. (vii) EF distributions were analyzed across individuals within each PPA variant and compared between groups. The left inferior parietal lobule received significantly more current in svPPA than nfvPPA (p < 0.05, Bonferroni corrected) with both frontal and dorsal montages (black box). In contrast, the left MTG exhibited lower current intensity in svPPA with the frontal montage (red box).. The name of each target ROI is prefixed with the kind of montage for which ACD values were obtained through EF modelling. For example, the name ‘D_L_Inferior Parietal_Lobule’ indicates ACD values at Left_IPL for the dorsal montage, ‘V_L_Inferior Parietal_Lobule’ indicates the ACD value for ventral montage, and similarly ‘F_L_Inferior Parietal_Lobule’ shows the ACD values for the frontal montage. Similar interpretations for the naming convention can be made for all 4 target ROIs across the three montages
Comparison between PPA groups
Atrophy common to both nfvPPA and svPPA was observed in the left inferior frontal orbitalis, bilateral insula, left fusiform, amygdala, caudate, putamen, and superior, middle, and inferior temporal gyri, including the superior temporal pole. Comparatively, nfvPPA showed greater atrophy in frontal areas, fusiform, and left insula, while svPPA had more atrophy in temporal regions, right insula, and left putamen (p < 0.05, Bonferroni corrected) (Fig. 1Sa). For entropy differences, only three regions overlapped between nfvPPA and svPPA—right amygdala, left middle temporal pole, and left cerebellum (Cerebellum_8)—where svPPA exhibited significantly higher atrophy (p < 0.05, Bonferroni corrected) (see supplement for details, Fig. 1Sb).
When comparing EF modelling across dorsal, ventral, and frontal montages, most target ROIs showed no significant differences. However, the left IPL received significantly more current in svPPA than nfvPPA (p < 0.05, Bonferroni corrected) with both frontal and dorsal montages, while the left MTG exhibited lower current intensity in svPPA with the frontal montage. As shown in Fig. 2(vii), the frontal region in nfvPPA, which undergoes significant atrophy, experiences lower current intensity, while a similar reduction is observed in the left MTG for svPPA, its primary atrophy site. These findings highlight the variant-specific impact of current distribution in PPA, reinforcing the need for customized tDCS montages tailored to pathology.
Correlation of atrophy and entropy with behaviours
Lastly, no significant correlation was found between atrophy and entropy measure with the cognition and language scores like Clinical Dementia Rating, Mini Mental State Exam, California Verbal Learning Test, Modified Trails, Digit Span, Verbal Fluency, Boston Naming Test (15-item), Montreal Cognitive Assessment, Peabody Picture Vocabulary Test, Geriatric Depression Scale, Pyramid Palm Tree Picture, for both the groups.
Table 1S and 2S in supplementary shows the mean as well as the standard deviation of these values (for both nfvPPA and svPPA) across all the significant cortical regions.
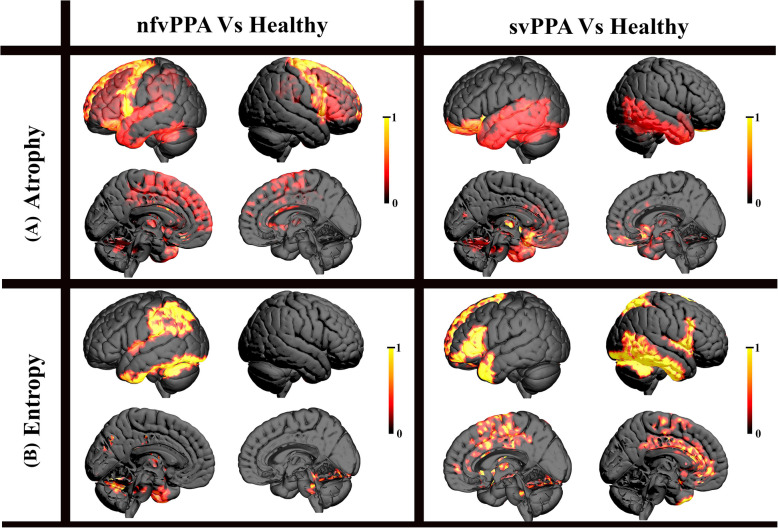
Figure 3 displays the cortical regions for which grey-matter volume and entropy were significantly different from healthy individuals for both the nfvPPA and svPPA groups. The areas that show significant reduction in grey matter volume are referred to as ‘atrophied’ areas. In the nfvPPA group, atrophy was prominent in both the left and right frontal regions, while in the svPPA, it was mostly left-dominant in the temporal region, with less severe loss in the right temporal region. Interestingly, the decrease in entropy occurred in regions distant from the atrophied areas. For instance, in nfvPPA, a significant decrease in entropy was observed in the parietal language regions as well as the few superior regions of the cerebellum. In contrast, in the svPPA variant, a significant decrease in entropy was seen in the left frontal region, the left anterior temporal pole, and most notably in the right temporal region. Additionally, significant involvement of medial and subcortical regions, such as the amygdala, caudate, and cingulum, was also observed for both variants. The detailed list of the regions involved can be found in Supplementary Table 1S and 2S.
Fig. 3.
shows the cortical regions that were significantly (p < 0.05, FDR corrected) different from healthy individuals for both the nfvPPA and svPPA groups in terms of (A) Atrophy and (B) Entropy. Anatomic distinction in the areas of atrophy and entropy can be observed
Association between atrophy, entropy and current parameters
For nfvPPA, the matrices (Number of subjects × Number of significant areas) for Y1 atrophy is of dimension 31 × 33 (where each entry represents the value of grey-matter volume of the areas significantly different from healthy), the Y2 entropy is of dimension 31 × 20 (where each entry represents the value of entropy of the brain areas significantly different from healthy), and the Y3 current matrix is of dimension 31 × 12 (where each entry is the ACD value at the target ROIs for each montage). For svPPA, the Y1 atrophy is 32 × 32 matrix, Y2 entropy matrix is 32 × 20, and the Y3 current matrix is of dimension 32 × 12. GAKCCA was used to estimate the multivariate relationship between current-intensity, atrophy and entropy matrices. When GAKCCA was applied to the nfvPPA and svPPA data separately using a permutation test with 10,000 iterations, significant relationships were found between atrophy (Y1) and entropy (Y2) for both the nfvPPA ( = 0.76, p = 0.005) and svPPA ( = 0.78, p = 0.01) groups. Similar significance was also reported between the atrophy (Y1) and current (Y3) for both the nfvPPA ( = 0.79, p = 0.01) and svPPA ( = 0.76, p = 0.01) groups. Since there was no significant association found between entropy (Y2) and current (Y3) for either nfvPPA ( = 0.40, p = 0.81) and svPPA ( = 0.60, p = 0.42) groups, these relationships are not discussed further (but are shown in the Figs. 4 (ii) and 5 (ii)). Covariance of Y1, Y2 and Y3 across the groups nfvPPA was
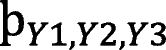 = 4.18 and svPPA is
= 4.18 and svPPA is
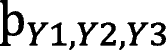 = 4.63.
= 4.63.
Fig. 4.
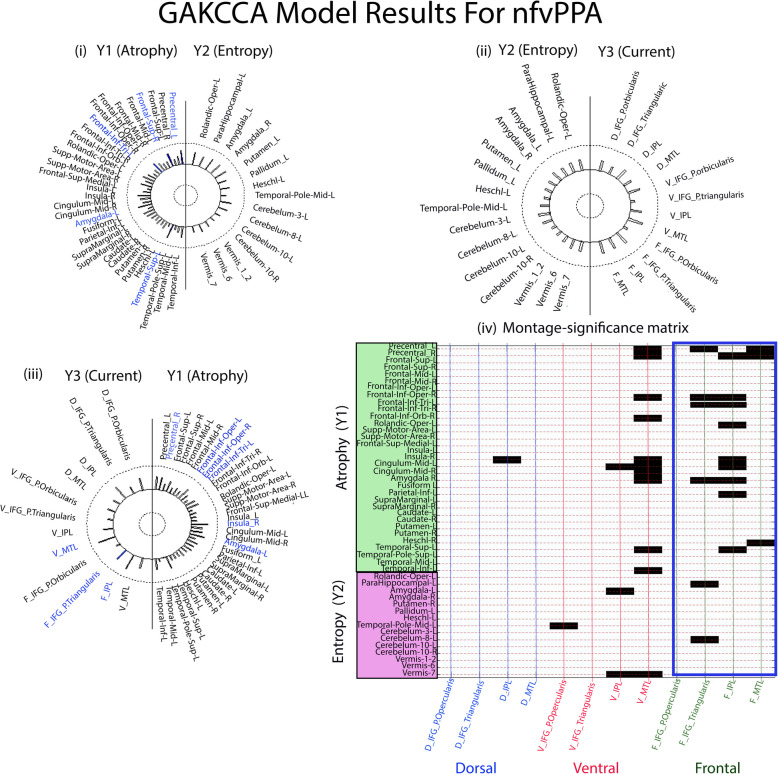
Helio plots of significant relationships between (i) atrophy (Y1) and entropy (Y2), (ii) entropy (Y2) and current (Y3), and (iii) current (Y3) and atrophy (Y1) based on the GAKCCA model for nfvPPA variant. The areas that significantly (p < 0.05) contribute to the association are highlighted in blue in the circles (iv) Montage-significance matrix showing significant correlations (p < 0.05, blocks shown in black) between the areas of atrophy and entropy with the ACD values of the target areas of each montage. Majority of the areas (black horizontal bars) showed significant association of the areas for frontal and ventral montage, though the count (number of areas) is higher in frontal montage marked in blue box (frontal montage n = 18, ventral montage n = 15, and dorsal montage n = 1)
Association between atrophy (Y1) and entropy (Y2)
For nfvPPA, atrophy in cortical regions ‘Precentral_L’ ( = 0.67, p = 0.01), ‘Frontal_Sup_L’ ( = 0.63, p = 0.01), ‘Frontal_Inf_Tri_L’ ( = 0.58, p = 0.04), ‘Amygdala_L’ ( = 0.58, p = 0.04) and ‘Temporal_Sup_L’ ( = 0.65, p = 0.02) are the significant contributor to the association of atrophy and entropy matrices (see Fig. 4(i)). Similarly, for the svPPA group, atrophy in regions- ‘Rectus_L’( = 0.53, p = 0.04), ‘Amygdala_R’ ( = 0.61, p = 0.02), ‘Occipital_Inf_L’ ( = 0.50, p = 0.04), ‘Putamen_R’ ( = 0.61, p = 0.01), ‘Temporal_Mid_R’ ( = 0.62, p = 0.01), ‘Temporal_Pole_Mid_R’ ( = 0.58, p = 0.02), and ‘Temp_Inf_R’ ( = 0.57, p = 0.05); and entropy in regions- ‘Supplemental_Motor_Area_R’ ( = 0.54, p = 0.04), ‘Amygdala_R’ ( = 0.54, p = 0.04), ‘Occipital_Inferior_R’ ( = 0.52, p = 0.04), and ‘Parietal_Superior_R’ ( = 0.51, p = 0.04) are the significant contributors to the association of atrophy with entropy (see Fig. 5(i)).
Fig. 5.
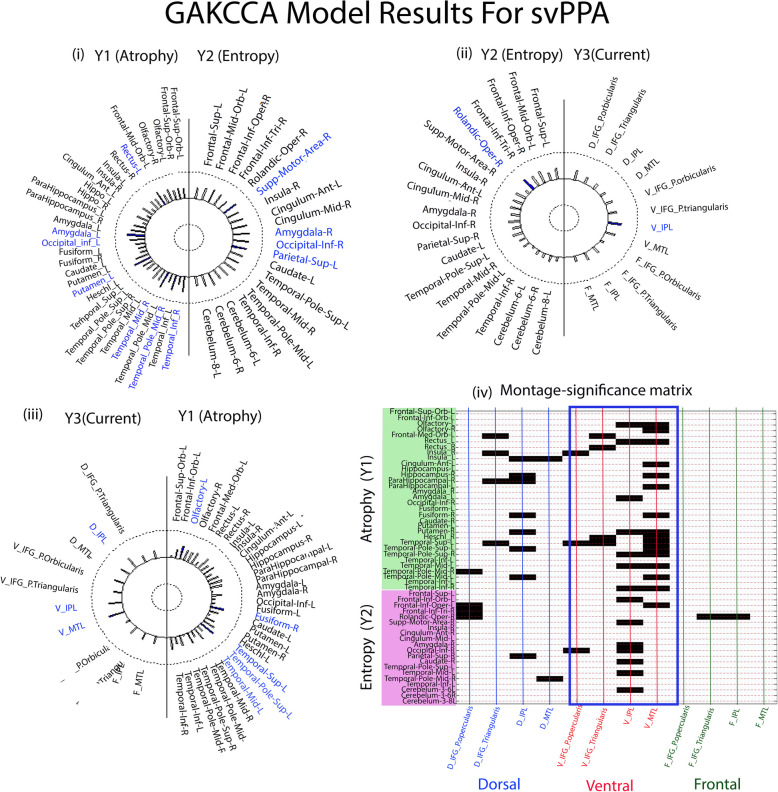
Helio plots of significant relationships between (i) atrophy (Y1) and entropy (Y2), (ii) entropy (Y2) and current (Y3), and (iii) current (Y3) and atrophy (Y1) based on the GAKCCA model for svPPA variant. The areas that significantly (p < 0.05) contribute to the association are highlighted in blue in the circles. (iv) Montage-significance matrix showing significant correlations (p < 0.05, blocks shown in black) between the areas of atrophy and entropy with the ACD values of target areas of each montage. Majority of the areas associated for ventral and dorsal montage, though the count (number of areas) is higher in ventral montage marked in blue box (ventral montage n = 37, dorsal montage n = 18, and frontal montage = 2)
Association between atrophy (Y1) and current (Y3)
For nfvPPA, atrophy in cortical regions ‘Precentral_L’ ( = 0.55, p = 0.04), ‘Frontal_Inf_Opercular_L’ ( = 0.50, p = 0.04), ‘Frontal_Inf_Opercular_R’ ( = 0.52, p = 0.04), ‘Frontal_Inf_Triangularis_L’ ( = 0.56, p = 0.04), ‘Insula_R’ ( = 0.64, p = 0.009), and ‘Amygdala_L’ ( = 0.56, p = 0.04), and ACD distribution in ‘V_L_MTG’ ( = 0.68, p = 0.03), ‘F_L_IPL’ ( = 0.65, p = 0.05), and ‘F_L_ IFG_P. Triangularis’ ( = 0.66, p = 0.03), are the significant contributors to the association between atrophy and current (Fig. 4(iii)).
For svPPA, atrophy in cortical regions ‘Olfactory_L’ ( = 0.47, p = 0.07), ‘Fusiform_R’ ( = 0.44, p = 0.07), ‘Temporal_Sup_L’ ( = 0.50, p = 0.04), ‘Temporal_pole_sup_L’ ( = 0.69, p = 0.02), and ‘Temporal_Mid_L’ ( = 0.73, p = 0.01), and ACD distribution in ‘V_L_MTG’ ( = 0.59, p = 0.04), ‘V_L_IPL’ ( = 0.58, p = 0.04), and ‘D_L_IPL’ ( = 0.56, p = 0.02) had significantly contributed to the association between atrophy and current (Fig. 5 (iii)).
Mapping of the montages with atrophy and entropy
For each montage, it is important to know how the current delivered to the target areas is in concordance with the areas that are atrophied and/or have altered entropy. To this, the montage-significance matrix was plotted for nfvPPA (Fig. 4(iv)). It was found that the ACD distribution in all 4 tDCS target ROIs following the dorsal montage stimulation was least associated with the areas of Y1 and Y2 matrix. In contrast, maximum ROIs of the frontal montage (n = 18) were significantly (p < 0.05) associated with areas of atrophy and entropy, followed by ROIs of the ventral montage (n = 15) (see Fig. 4(iv) for details).
For svPPA, when the montage-significance matrix was plotted, it was found that the ACD distribution in all four ROIs following the frontal montage configuration was least associated with the areas of Y1 and Y2 matrix. In contrast, maximum ROIs of the ventral (n = 37) were significantly (p < 0.05) associated with areas of atrophy and entropy, followed by ROIs of dorsal montages (n = 18) (see Fig. 5 (iv) for details).
Additional Analysis
After establishing the association between atrophy and entropy with current at target ROIs across the three montage simulations, it is essential to verify whether key language-related regions are effectively targeted. To assess this, we correlated CD at target ROIs with the Dose Target Determination Index (DTDI)—a measure from our previous work [7, 13] that quantifies the probability of delivering peak current to the target ROI relative to non-target regions (supplement section 5 for details). In our selected four language-related hubs as target ROIs, a significant correlation between CD and DTDI (Fig. 2S) confirms that higher current intensity at the target ROIs increases the likelihood of peak stimulation. This suggests that atrophied regions associated with CD at target ROIs play a role in determining the probability of delivering peak current, ultimately impacting the effectiveness of target engagement.
Lastly, Sex plays a very important role in determination of pathology specific montages [6]. To address thiswe performed the GAKCCA analysis independently for male and female groups as performed in prior studies [71–74] and found no differences based on sex. The associations identified in the total sample remained significant in both genders (refer supplement-section 6).
Discussion
This study modelled the association between the distribution of tDCS current in language-specific brain regions with the structural (atrophy) and functional (entropy) characteristics of regions impaired in the two variants of PPA. The aim was to determine the suitability of tDCS montage for nfvPPA and svPPA in alignment with the brain’s intrinsic functional and structural organisation. The study revealed three main findings: (1) In the PPA group, all three parameters—grey-matter volume, entropy, and current-intensity at target ROIs of language network—decreased compared to healthy individuals. As expected, PPA patients showed more atrophy than healthy subjects. The current at target ROIs was significantly related to atrophy but not to entropy, although atrophy and entropy were significantly associated with each other. (2) Areas of atrophy and entropy were distinct in their anatomic localisation- Significant atrophy was observed in the left-frontal regions for the nfvPPA variant and in the left-temporal region for the svPPA variant. For entropy, a decrease was noted in the left-parietal and few cerebellar regions in the nfvPPA variant, and in the left-frontal and right-temporal regions in the svPPA variant. (3) In the nfvPPA variant, the current distribution at target ROIs following a dorsal montage was least associated with changes in regional atrophy or entropy, while the frontal montage showed the strongest association (also ventral montage to certain extent). In the svPPA variant, the current distribution in target ROIs showed the weakest association in frontal montage, while the ventral montage showed the strongest association with the areas with significant variation in atrophy and entropy.
Brain atrophy typically decreases tDCS current-intensity at the target ROI and is crucial for optimizing neurostimulation protocols in neurodegenerative conditions [75]. Atrophy reduces brain volume and alters tissue composition, impacting electrical conductivity and potentially varying the tDCS effects [5, 6, 21, 61]. The influence of atrophy patterns on the current flow is a determinant in the placement of tDCS electrodes on the scalp [35, 36, 76]. For safe and effective simulation, computational models have stressed the importance of adjusting the montages based on the atrophy pattern of an individual [6, 77]. Moreover, atrophic regions can alter cortical excitability, affecting tDCS efficacy in modulating neural activity and cognitive functions. Therefore, pathology-specific personalization of the protocols using advanced imaging is seen as the next frontier that can optimize tDCS outcomes based on the underlying organisation of an individual’s brain [4, 39].
To our knowledge, this is the first study to show that the influence of tDCS montages on current distribution and atrophy varies by PPA variant. In nfvPPA, frontal montage stimulation is strongly associated with frontal atrophy, while in svPPA, ventral montage stimulation correlates with temporal atrophy. Conversely, nfvPPA shows the weakest association with dorsal montage, and svPPA with frontal montage. These findings align with expectations, as electrode proximity to target regions naturally directs more current into atrophied areas, making them more sensitive due to increased CSF conductivity. Previous studies indicate that CSF pockets between target and reference electrodes affect tDCS focality, with closer CSF pockets increasing current penetration into the target, while distant CSF pockets divert current away [7]. For the first time in PPA patients, we validate prior findings from healthy individuals linking brain atrophy, current density, and electrode placement [7, 75]. The relationship between atrophy and current distribution is key to selecting an effective tDCS montage, as aligning stimulation with atrophied regions enhances neural excitability and therapeutic impact. Moreover, the stronger association between atrophy in multiple regions and current distribution suggests that atrophy plays a critical role in effective target engagement.
We found no association between entropy and simulated tDCS current distribution, but a strong link between regional atrophy and entropy. A study on 862 healthy adults showed that entropy in the default mode and executive control networks increases with age but decreases with education, a marker of cognitive reserve [9, 50]. Lower entropy in these networks correlated with better cognitive function, highlighting entropy as a potential indicator of latent brain reserve compensation. Only one prior study differentiated entropy patterns between Alzheimer’s disease and frontotemporal dementia using machine-learning feature extraction, particularly in early stages prone to misdiagnosis [78]. Our study is the first to report that frontal and temporal atrophy in nfvPPA and svPPA may lead to distinct entropy changes, reflecting disease-specific neural alterations. Typically, atrophy and entropy reductions occur in the same regions, as brain tissue loss reduces neural complexity and adaptability [79]. However, compensatory mechanisms may cause entropy changes in unaffected regions, reflecting neural adaptation [80].
Emerging research suggests that entropy reduction influences tDCS efficacy beyond traditional EF modeling. Studies indicate that reduced entropy enhances executive control following tDCS [81], informs personalized tDCS applications [82], and affects seizure susceptibility in epilepsy following tDCS [83]. Additionally, entropy-based modulation has been linked to longer-lasting neuroplastic changes in tDCS induced stroke rehabilitation [84], minimally conscious patients [47], and depression treatment [85]. Machine learning approaches incorporating entropy-based neural markers have improved tDCS treatment predictions beyond EF modeling alone [86]. These findings highlight entropy as a key marker of dynamic network states, where reductions indicate increased synchrony and neuroplasticity. Integrating entropy-based analyses with EF models could further refine tDCS protocols, optimizing their clinical efficacy.
Altogether, we examined how the current distribution in targeted language ROIs for each montage interacts with areas showing significant changes in atrophy and entropy. We found that the tDCS montage most associated with changes in atrophy was distant from regions with altered entropy, and this relationship varied by PPA variant. In nfvPPA, the frontal montage’s current distribution was influenced mostly by atrophy, while areas with entropy changes remained distant. Similarly, in svPPA, the ventral montage’s current distribution was driven by atrophy, and the entropy-altered left-frontal regions remained distant. It is true that ACD calculations, derived from computational modelling using structural brain scans, contain anatomical information about the brain’s structure. Our analysis deepens the association between Y1 (atrophy) and Y3 (current) to highlight the necessity of selecting atrophy-specific stimulation parameters in PPA. One of the key focuses of this study was to simulate three distinct electrode montages (targeting specific areas of the language network) for the two PPA variants and identify the montages that maximized (or minimized) the association with atrophied regions. The findings provided the benchmark support to claim that instead of applying a standard (e.g., frontal) montage for all PPA patients, electrode placement should be tailored to each variant-specific atrophy pattern.
Limitations and future implications
This study has limitations, primarily relying on structural–functional-EF modelling of brain images; experimental validation is needed to confirm these findings. While in-vivo intracortical recordings align with the simulated current [14], validation of EF modelling in atrophied brains remains crucial. Our approach to tailoring montages by PPA variant is a shift from the standard uniform montage (e.g., frontal) across all PPA types, aiming for targeted rehabilitation benefits. While empirical validation is essential, this computational groundwork supports initiating a clinical trial with a novel approach. Lastly, the study excluded heterogeneous logopenic and mixed-variant PPA cases. This was because the NIFD dataset did not include these two variants in their data collection. However, this framework could be expanded to create atrophy-specific tDCS protocols across all PPA variants.
Since tDCS aims to restore function in impaired brain areas [87], findings of present study suggest that in nfvPPA and svPPA, frontal and ventral montages are more suitable in future trials with tDCS. This supports a recent clinical trial that found nfvPPA benefited from frontal montage tDCS, while svPPA showed less improvement with the same treatment [9]. It appears that targeting atrophy-related regions (frontal for nfvPPA) is likely to be more effective than targeting areas with decreased entropy (frontal for svPPA). Another key observation for nfvPPA is the comparable suitability of ventral and frontal montages, as the number of affected areas is roughly similar. This is important, as the effectiveness of montage selection depends on the task being targeted [88]. For example, the same trial [10] reported frontal montage was more successful for retrieving trained items’ orthographic forms but less effective for untrained items requiring improved semantics. If our approach were integrated, it could suggest that targeting the MTG with a ventral montage may be better suited to enhance semantic abilities. Moreover, we observed that the dorsal montage showed the weakest association with atrophy in nfvPPA, indicating it may be less recommended. This aligns with the fact that the temporoparietal junction—targeted by the dorsal montage—is relatively spared in nfvPPA [8]. Experimental verification is needed, but it suggests that dorsal montage may be less suitable for nfvPPA. Similarly, we anticipate that dorsal and ventral montages will benefit svPPA patients more than frontal montages, with dorsal stimulation potentially aiding patients with naming difficulties but intact comprehension, and ventral stimulation benefiting those with semantic loss.
Additionally, as neurodegeneration progresses, the effectiveness of pathology-specific tDCS montages may change. While our study identifies optimal montages based on current atrophy patterns, longitudinal studies are needed to assess the benefits of adaptive tDCS protocols. Periodic MRI-based reassessment could guide dynamic adjustments, ensuring effective stimulation protocols can be made available to patients at different stages during disease progression.
Lastly, entropy was chosen as a functional measure because it captures brain signal complexity and variability, offering a global assessment of neural adaptability in PPA. Unlike functional or structural connectivity measures, which focus on specific network interactions, entropy provides a broader view of signal dynamics, making it particularly useful for detecting disruptions in spontaneous neural activity. However, it has limitations, including a lack of network-specific insights and the inability to map region-based connectivity. Additionally, as observed in this study, entropy alone may not directly predict cognitive performance. Future research integrating entropy with connectivity analyses could offer a more comprehensive understanding of functional and structural changes in PPA.
While our study focuses on grey matter atrophy, white matter degeneration may also impact tDCS efficacy by altering current distribution and focality. Integrating DTI-based connectivity with EF modelling could further enhance individualized tDCS strategies for PPA.
Conclusion
This study examined how tDCS current distribution relates to atrophy and entropy in nfvPPA and svPPA. Though the distribution atrophy and entropy vary by PPA variant, atrophy predominantly guides the distribution of current in language areas due to a montage. This emphasizes the need to use atrophy-specific tDCS montages (frontal montage for nfvPPA and ventral montage for svPPA) to stimulate the affected areas in each variant of PPA.
Supplementary Information
Acknowledgements
We appreciate the assistance of Eunseong Bae, a PhD candidate in the Department of Statistics at the University of California, Davis, USA, for aiding us in the implementation and interpretation of the GAKCCA statistical technique in our manuscript. SB and RK are funded by the DBT Ramalingaswami re-entry fellowship from the Government of India (D.O NO BT/HRD/35/02/2006). Additionally, JD received support from the NIH/NICHD grant P50 HD103538. The funding body had no involvement in the study design, data collection, analysis, interpretation, report writing, or the decision to submit the article for publication.
Authors’ contributions
SB contributed to the conceptualization, formal analysis, funding, writing, and revising of the original draft. She was in charge of the study overall. RK contributed to conceptualization, managing resources and software, technical guidance, supervision, and validation. PTS, GV, KO, KT, AC, BR, JD, TNS, and KU contributed to writing—review and editing.
Funding
SB and RK are funded by the DBT Ramalingaswami re-entry fellowship from the Government of India (D.O NO BT/HRD/35/02/2006). Additionally, JD received support from the NIH/NICHD grant P50 HD103538. The funding body had no involvement in the study design, data collection, analysis, interpretation, report writing, or the decision to submit the article for publication.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The present study is approved by the National Institute of Mental Health and Neuro Sciences (NIMHANS) ethical review board [NIMHANS/39 th IEC (BS & NS DIV.)/].
Competing interests
The authors declare no competing interests.
Footnotes
Sagarika Bhattacharjee is the senior author.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sagarika Bhattacharjee, Email: bhattacharya.sagarika7@gmail.com.
Rajan Kashyap, Email: rajankashyap6@gmail.com.
References
- 1.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesulam M. Primary progressive aphasia: A dementia of the language network. Dement Neuropsychol. 2013;7:2–9. 10.1590/S1980-57642013DN70100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesulam M-M, Weintraub S. Is it time to revisit the classification guidelines for primary progressive aphasia? Neurology. 2014;82:1108–9. [DOI] [PubMed] [Google Scholar]
- 4.Coemans S, Struys E, Vandenborre D, Wilssens I, Engelborghs S, Paquier P, et al. A Systematic Review of Transcranial Direct Current Stimulation in Primary Progressive Aphasia: Methodological Considerations. Front Aging Neurosci 2021;13. 10.3389/fnagi.2021.710818. [DOI] [PMC free article] [PubMed]
- 5.Kashyap R, Bhattacharjee S, Arumugam R, Bharath RD, Udupa K, Oishi K, Guan C. Focality-oriented selection of current dose for transcranial direct current stimulation. J Pers Med. 2021;11(9):940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharjee S, Kashyap R, Goodwill AM, O’Brien BA, Rapp B, Oishi K, et al. Sex difference in tDCS current mediated by changes in cortical anatomy: A study across young, middle and older adults. Brain Stimul. 2022;15:125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashyap R, Bhattacharjee S, Bharath RD, Venkatasubramanian G, Udupa K, Bashir S, et al. Variation of cerebrospinal fluid in specific regions regulates focality in transcranial direct current stimulation. Front Hum Neurosci. 2022;16:952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesulam M-M, Coventry CA, Rader BM, Kuang A, Sridhar J, Martersteck A, et al. Modularity and granularity across the language network-a primary progressive aphasia perspective. Cortex. 2021;141:482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsapkini K, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, et al. Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimer’s Dementia. 2018;4:461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Aguiar V, Zhao Y, Faria A, Ficek B, Webster KT, Wendt H, et al. Brain volumes as predictors of tDCS effects in primary progressive aphasia. Brain Lang. 2020;200:104707. 10.1016/j.bandl.2019.104707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ficek BN, Wang Z, Zhao Y, Webster KT, Desmond JE, Hillis AE, et al. The effect of tDCS on functional connectivity in primary progressive aphasia. NeuroImage: Clinical 2018;19:703–15. [DOI] [PMC free article] [PubMed]
- 12.Datta A, Baker JM, Bikson M, Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul. 2011;4:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashyap R, Bhattacharjee S, Arumugam R, Oishi K, Desmond JE, Chen SA. i-SATA: A MATLAB based toolbox to estimate current density generated by transcranial direct current stimulation in an individual brain. J Neural Eng. 2020;17:056034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X, Parra LC. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. elife. 2017;6:e18834. [DOI] [PMC free article] [PubMed]
- 15.Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage. 2013;74:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song D, Chang D, Zhang J, Peng W, Shang Y, Gao X, et al. Reduced brain entropy by repetitive transcranial magnetic stimulation on the left dorsolateral prefrontal cortex in healthy young adults. Brain Imaging Behav. 2019;13:421–9. 10.1007/s11682-018-9866-4. [DOI] [PubMed] [Google Scholar]
- 17.Antonenko D, Grittner U, Saturnino G, Nierhaus T, Thielscher A, Flöel A. Inter-individual and age-dependent variability in simulated electric fields induced by conventional transcranial electrical stimulation. Neuroimage. 2021;224:117413. 10.1016/j.neuroimage.2020.117413. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C, Datta A, Woods A. Effect of aging on cortical current flow due to transcranial direct current stimulation: considerations for safety. 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE; 2018, p. 3084–7. [DOI] [PubMed]
- 19.Mahdavi S, Towhidkhah F, Initiative ADN. Computational human head models of tDCS: influence of brain atrophy on current density distribution. Brain Stimul. 2018;11:104–7. [DOI] [PubMed] [Google Scholar]
- 20.Unal G, Ficek B, Webster K, Shahabuddin S, Truong D, Hampstead B, et al. Impact of brain atrophy on tDCS and HD-tDCS current flow: a modeling study in three variants of primary progressive aphasia. Neurol Sci. 2020;41:1781–9. 10.1007/s10072-019-04229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharjee S, Kashyap R, Rapp B, Oishi K, Desmond JE, Chen SHA. Simulation Analyses of tDCS Montages for the Investigation of Dorsal and Ventral Pathways. Sci Rep. 2019;9:12178. 10.1038/s41598-019-47654-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshmiri S. Entropy and the Brain: An Overview. Entropy 2020;22. 10.3390/e22090917. [DOI] [PMC free article] [PubMed]
- 23.Wang B, Niu Y, Miao L, Cao R, Yan P, Guo H, et al. Decreased complexity in Alzheimer’s disease: resting-state fMRI evidence of brain entropy mapping. Front Aging Neurosci. 2017;9:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau ZJ, Pham T, Chen SHA, Makowski D. Brain entropy, fractal dimensions and predictability: A review of complexity measures for EEG in healthy and neuropsychiatric populations. Eur J of Neurosci. 2022;56:5047–69. 10.1111/ejn.15800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alkire MT, Hudetz AG, Tononi G. Consciousness and Anesthesia. Science. 2008;322:876–80. 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagerholm ED, Scott G, Shew WL, Song C, Leech R, Knöpfel T, et al. Cortical Entropy, Mutual Information and Scale-Free Dynamics in Waking Mice. Cereb Cortex. 2016;26:3945–52. 10.1093/cercor/bhw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olofsen E, Sleigh JW, Dahan A. Permutation entropy of the electroencephalogram: a measure of anaesthetic drug effect. BJA: British Journal of Anaesthesia 2008;101:810–21. 10.1093/bja/aen290. [DOI] [PubMed]
- 28.Silva A, Cardoso-Cruz H, Silva F, Galhardo V, Antunes L. Comparison of Anesthetic Depth Indexes Based on Thalamocortical Local Field Potentials in Rats. Anesthesiology. 2010;112:355–63. 10.1097/ALN.0b013e3181ca3196. [DOI] [PubMed] [Google Scholar]
- 29.Atasoy S, Roseman L, Kaelen M, Kringelbach ML, Deco G, Carhart-Harris RL. Connectome-harmonic decomposition of human brain activity reveals dynamical repertoire re-organization under LSD. Sci Rep. 2017;7:17661. 10.1038/s41598-017-17546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carhart-Harris RL. The entropic brain - revisited. Neuropharmacology. 2018;142:167–78. 10.1016/j.neuropharm.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Carhart-Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 2014;8. 10.3389/fnhum.2014.00020. [DOI] [PMC free article] [PubMed]
- 32.Lebedev A v., Kaelen M, Lövdén M, Nilsson J, Feilding A, Nutt D j., et al. LSD-induced entropic brain activity predicts subsequent personality change. Human Brain Mapping 2016;37:3203–13. 10.1002/hbm.23234. [DOI] [PMC free article] [PubMed]
- 33.Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK, Muthukumaraswamy SD. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine. LSD and psilocybin Sci Rep. 2017;7:46421. 10.1038/srep46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viol A, Palhano-Fontes F, Onias H, de Araujo DB, Viswanathan GM. Shannon entropy of brain functional complex networks under the influence of the psychedelic Ayahuasca. Sci Rep. 2017;7:7388. 10.1038/s41598-017-06854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M, Liu X, Hildebrandt A, Zhou C. Individual Cortical Entropy Profile: Test–Retest Reliability, Predictive Power for Cognitive Ability, and Neuroanatomical Foundation. Cerebral Cortex Communications 2020;1:tgaa015. 10.1093/texcom/tgaa015. [DOI] [PMC free article] [PubMed]
- 36.Saxe GN, Calderone D, Morales LJ. Brain entropy and human intelligence: A resting-state fMRI study. PLoS ONE. 2018;13:e0191582. 10.1371/journal.pone.0191582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutchison RM, Morton JB. Tracking the Brain’s Functional Coupling Dynamics over Development. J Neurosci. 2015;35:6849–59. 10.1523/JNEUROSCI.4638-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou W, Wang D, Wong A, Chu WCW, Mok VCT, Shi L. Frequency-specific age-related decreased brain network diversity in cognitively healthy elderly: A whole-brain data-driven analysis. Hum Brain Mapp. 2019;40:340–51. 10.1002/hbm.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McIntosh AR, Kovacevic N, Lippe S, Garrett D, Grady C, Jirsa V. The Development of a Noisy Brain. Arch Ital Biol. 2010;148:323–37. 10.4449/aib.v148i3.1225. [PubMed] [Google Scholar]
- 40.McIntosh AR, Kovacevic N, Itier RJ. Increased Brain Signal Variability Accompanies Lower Behavioral Variability in Development. PLoS Comput Biol. 2008;4:e1000106. 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang AC, Huang C-C, Yeh H-L, Liu M-E, Hong C-J, Tu P-C, et al. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: a multiscale entropy analysis. Neurobiol Aging. 2013;34:428–38. [DOI] [PubMed] [Google Scholar]
- 42.Nascimento DC, Depetri G, Stefano LH, Anacleto O, Leite JP, Edwards DJ, et al. Entropy analysis of high-definition transcranial electric stimulation effects on eeg dynamics. Brain Sci. 2019;9:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodranghien FC, Langlois Mahe M, Clément S, Manto MU. A pilot study on the effects of transcranial direct current stimulation on brain rhythms and entropy during self-paced finger movement using the epoc helmet. Front Hum Neurosci. 2017;11:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou D, Zhou J, Chen H, Manor B, Lin J, Zhang J. Effects of transcranial direct current stimulation (tDCS) on multiscale complexity of dual-task postural control in older adults. Exp Brain Res. 2015;233:2401–9. 10.1007/s00221-015-4310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang J, Cai E, Han J, Tong Z, Li X, Sokhadze EM, Li X. Transcranial direct current stimulation (tDCS) can modulate EEG complexity of children with autism spectrum disorder. Front Neurosci. 2018;12:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miraglia F, Pappalettera C, Barbati SA, Podda MV, Grassi C, Rossini PM, et al. Brain complexity in stroke recovery after bihemispheric transcranial direct current stimulation in mice. Brain Communications 2024;6:fcae137. [DOI] [PMC free article] [PubMed]
- 47.Martens G, Ibáñez-Soria D, Barra A, Soria-Frisch A, Piarulli A, Gosseries O, et al. A novel closed-loop EEG-tDCS approach to promote responsiveness of patients in minimally conscious state: a study protocol. Behav Brain Res. 2021;409:113311. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharjee S, Kashyap R, Rapp B, Oishi K, Desmond JE, Chen SA. Simulation analyses of tDCS montages for the investigation of dorsal and ventral pathways. Sci Rep. 2019;9:12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsapkini K, Frangakis C, Gomez Y, Davis C, Hillis AE. Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: preliminary results and challenges. Aphasiology. 2014;28:1112–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Aguiar V, Zhao Y, Ficek BN, Webster K, Rofes A, Wendt H, et al. Cognitive and language performance predicts effects of spelling intervention and tDCS in Primary Progressive Aphasia. Cortex. 2020;124:66–84. 10.1016/j.cortex.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhattacharjee S, Chew A, Kashyap R, Wu C, Yeo M, O’Brien B, Chen S. Could tDCS modulate bilingual reading?. Brain Stimul: Basic, Transl, Clin Res Neuromodulation. 2019;12(2):569. [Google Scholar]
- 52.Bhattacharjee S, Kashyap R, O’Brien BA, McCloskey M, Oishi K, Desmond JE, et al. Reading proficiency influences the effects of transcranial direct current stimulation: Evidence from selective modulation of dorsal and ventral pathways of reading in bilinguals. Brain Lang. 2020;210:104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fenner AS, Webster KT, Ficek BN, Frangakis CE, Tsapkini K. Written verb naming improves after tDCS over the left IFG in primary progressive aphasia. Front Psychol. 2019;10:1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris AD, Wang Z, Ficek B, Webster K, Edden RA, Tsapkini K. Reductions in GABA following a tDCS-language intervention for primary progressive aphasia. Neurobiol Aging. 2019;79:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Licata AE, Zhao Y, Herrmann O, Hillis AE, Desmond J, Onyike C, et al. Sex differences in effects of tDCS and language treatments on brain functional connectivity in primary progressive aphasia. NeuroImage: Clinical 2023;37:103329. 10.1016/j.nicl.2023.103329. [DOI] [PMC free article] [PubMed]
- 56.Huang Y, Datta A, Bikson M, Parra LC. Realistic volumetric-approach to simulate transcranial electric stimulation—ROAST—a fully automated open-source pipeline. J Neural Eng. 2019;16:056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. [DOI] [PubMed] [Google Scholar]
- 58.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. [DOI] [PubMed] [Google Scholar]
- 59.Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020;206:116189. [DOI] [PubMed] [Google Scholar]
- 60.SPM - Statistical Parametric Mapping n.d. https://www.fil.ion.ucl.ac.uk/spm/ (accessed July 4, 2019).
- 61.Kashyap R, Bhattacharjee S, Yeo BT, Chen SA. Maximizing dissimilarity in resting state detects heterogeneous subtypes in healthy population associated with high substance use and problems in antisocial personality. Hum Brain Mapp. 2020;41:1261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kashyap R, Kong R, Bhattacharjee S, Li J, Zhou J, Yeo BT. Individual-specific fMRI-Subspaces improve functional connectivity prediction of behavior. Neuroimage. 2019;189:804–12. [DOI] [PubMed] [Google Scholar]
- 63.Kashyap R, Eng GK, Bhattacharjee S, Gupta B, Ho R, Ho CS, Chen SA. Individual-fMRI-approaches reveal cerebellum and visual communities to be functionally connected in obsessive compulsive disorder. Sci Rep. 2021;11(1):1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costa M, Goldberger AL, Peng C-K. Multiscale entropy analysis of biological signals. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:021906. 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- 65.Yang AC, Tsai SJ, Lin CP, Peng CK. A strategy to reduce bias of entropy estimates in resting-state fMRI signals. Front neurosci. 2018;12:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costa M, Goldberger AL, Peng C-K. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z. The neurocognitive correlates of brain entropy estimated by resting state fMRI. Neuroimage. 2021;232:117893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sokunbi MO, Fung W, Sawlani V, Choppin S, Linden DE, Thome J. Resting state fMRI entropy probes complexity of brain activity in adults with ADHD. Psychiatry Res. 2013;214:341–8. [DOI] [PubMed] [Google Scholar]
- 69.Sokunbi MO, Gradin VB, Waiter GD, Cameron GG, Ahearn TS, Murray AD, et al. Nonlinear complexity analysis of brain FMRI signals in schizophrenia. PLoS ONE. 2014;9:e95146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bae E, Hur J-W, Kim J, Kwon JS, Lee J, Lee S-H, et al. Multi-group analysis using generalized additive kernel canonical correlation analysis. Sci Rep. 2020;10:12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parazzini M, Fiocchi S, Ravazzani P. Electric field and current density distribution in an anatomical head model during transcranial direct current stimulation for tinnitus treatment. Bioelectromagnetics. 2012;33:476–87. [DOI] [PubMed] [Google Scholar]
- 72.Parazzini M, Fiocchi S, Rossi E, Paglialonga A, Ravazzani P. Transcranial direct current stimulation: estimation of the electric field and of the current density in an anatomical human head model. IEEE Trans Biomed Eng. 2011;58:1773–80. [DOI] [PubMed] [Google Scholar]
- 73.Parazzini M, Fiocchi S, Liorni I, Priori A, Ravazzani P. Computational modeling of transcranial direct current stimulation in the child brain: implications for the treatment of refractory childhood focal epilepsy. Int J Neural Syst. 2014;24:1430006. [DOI] [PubMed] [Google Scholar]
- 74.Vergallito A, Feroldi S, Pisoni A, Romero Lauro LJ. Inter-individual variability in tDCS effects: A narrative review on the contribution of stable, variable, and contextual factors. Brain Sci. 2022;12:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Habich A, Fehér KD, Antonenko D, Boraxbekk C-J, Flöel A, Nissen C, et al. Stimulating aged brains with transcranial direct current stimulation: opportunities and challenges. Psychiatry Research. 2020;306:111179. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharjee S, Kashyap R, Sreeraj VS, Sivakumar PT, Venkatasubramanian G, Desmond JE, Udupa K. Personalized Dose Selection for Treatment of Patients with Neuropsychiatric Disorders Using tDCS. Brain Sci. 2024;14(12):1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Indahlastari A, Albizu A, O’Shea A, Forbes MA, Nissim NR, Kraft JN, Alzheimer’s Disease Neuroimaging Initiative. Modeling transcranial electrical stimulation in the aging brain. Brain stimul. 2020;13(3):664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lal U, Chikkankod AV, Longo L. Leveraging SVD Entropy and Explainable Machine Learning for Alzheimer’s and Frontotemporal Dementia Detection using EEG 2023. 10.36227/techrxiv.23992554.v2.
- 79.Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67:1340–52. 10.1212/01.wnl.0000240127.89601.83. [DOI] [PubMed] [Google Scholar]
- 80.Xue S-W, Guo Y, Initiative ADN. Increased resting-state brain entropy in Alzheimer’s disease. NeuroReport. 2018;29:286–90. [DOI] [PubMed] [Google Scholar]
- 81.Alexandersen A, Csifcsák G, Groot J, Mittner M. The effect of transcranial direct current stimulation on the interplay between executive control, behavioral variability and mind wandering: A registered report. Neuroimage: Reports 2022;2:100109. 10.1016/j.ynirp.2022.100109. [DOI] [PMC free article] [PubMed]
- 82.Beumer S, Boon P, Klooster DCW, van Ee R, Carrette E, Paulides MM, et al. Personalized tDCS for Focal Epilepsy—A Narrative Review: A Data-Driven Workflow Based on Imaging and EEG Data. Brain Sci. 2022;12:610. 10.3390/brainsci12050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ali MM, Sellers KK, Fröhlich F. Transcranial Alternating Current Stimulation Modulates Large-Scale Cortical Network Activity by Network Resonance. J Neurosci. 2013;33:11262–75. 10.1523/JNEUROSCI.5867-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pappalettera C. Transcranial direct current stimulation for rehabilitation in stroke sufferers with upper limb deficit 2025.
- 85.Borrione L, Bellini H, Razza LB, Avila AG, Baeken C, Brem A-K, et al. Precision non-implantable neuromodulation therapies: a perspective for the depressed brain. Braz J Psychiatry. 2020;42:403–19. 10.1590/1516-4446-2019-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Albizu A, Indahlastari A, Huang Z, Waner J, Stolte SE, Fang R, et al. Machine-learning defined precision tDCS for improving cognitive function. Brain Stimul. 2023;16:969–74. 10.1016/j.brs.2023.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bikson M, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci 2013;7. 10.3389/fnhum.2013.00688. [DOI] [PMC free article] [PubMed]
- 88.Bhattacharjee S, Kashyap R, Udupa K, Bashir S, Venkatsubramanian G, Oishi K, et al. Alignment of behaviour and tDCS stimulation site induces maximum response: evidence from online tDCS and ERP. Sci Rep. 2024;14:19715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.