Abstract
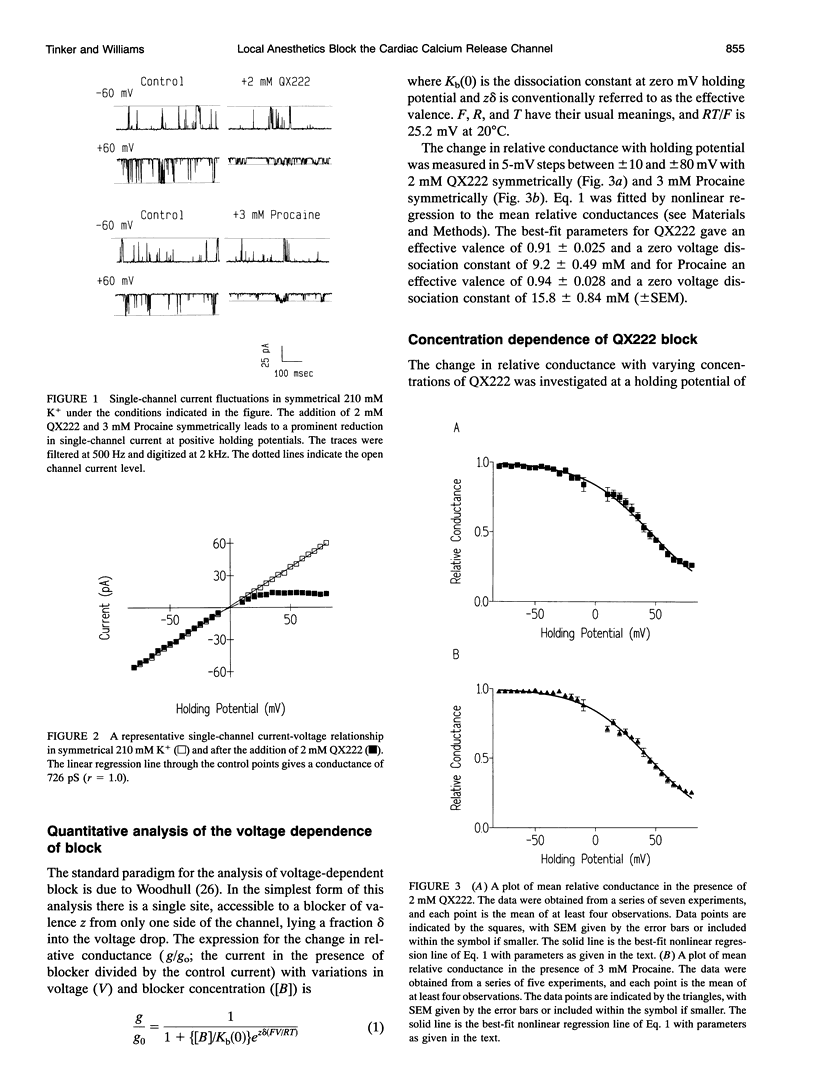
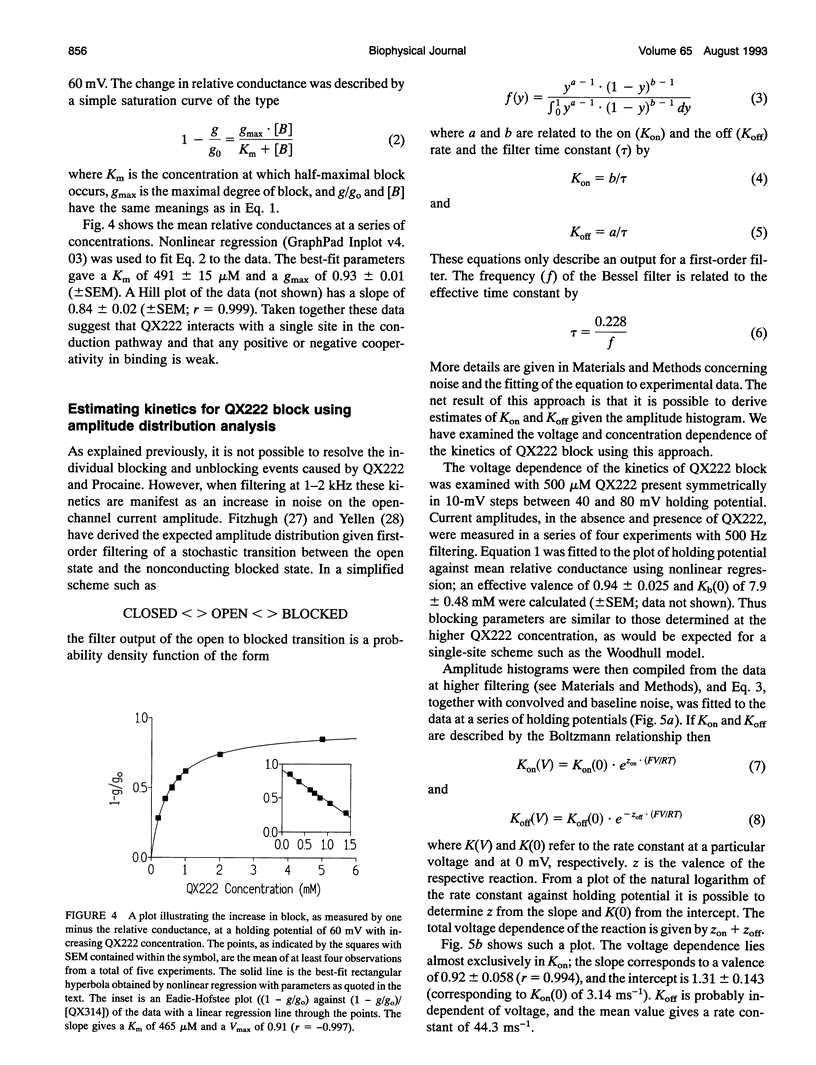
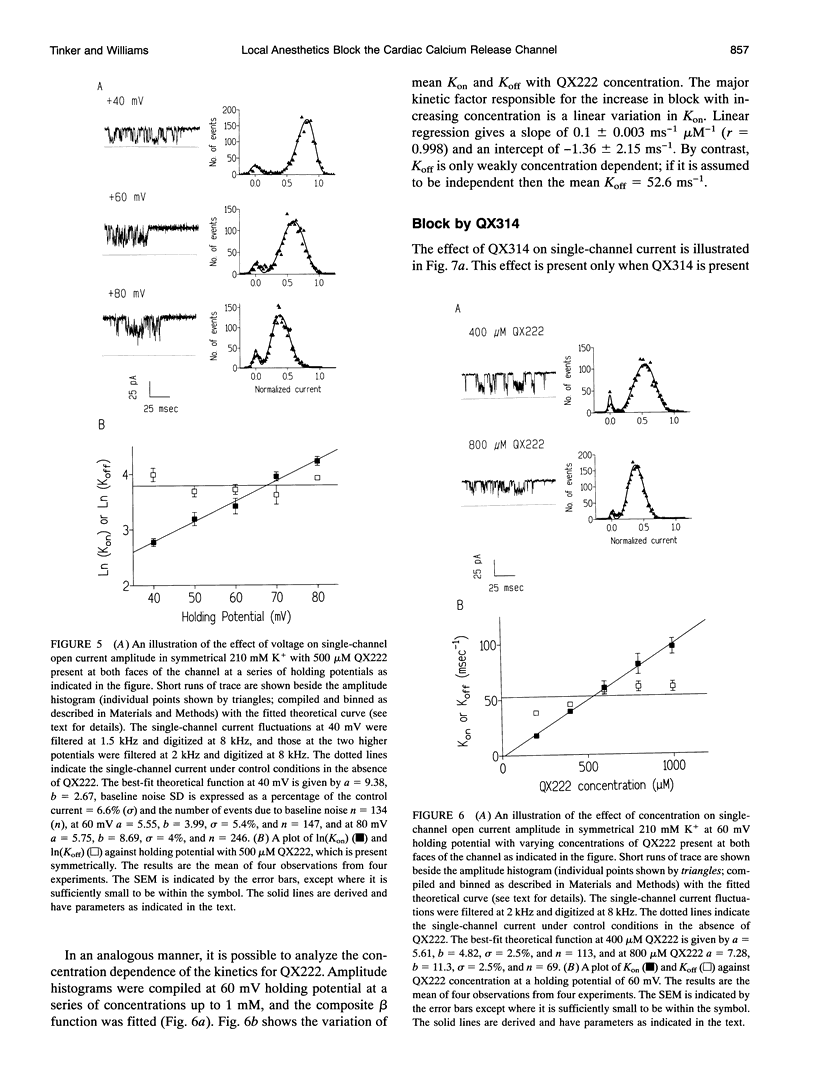
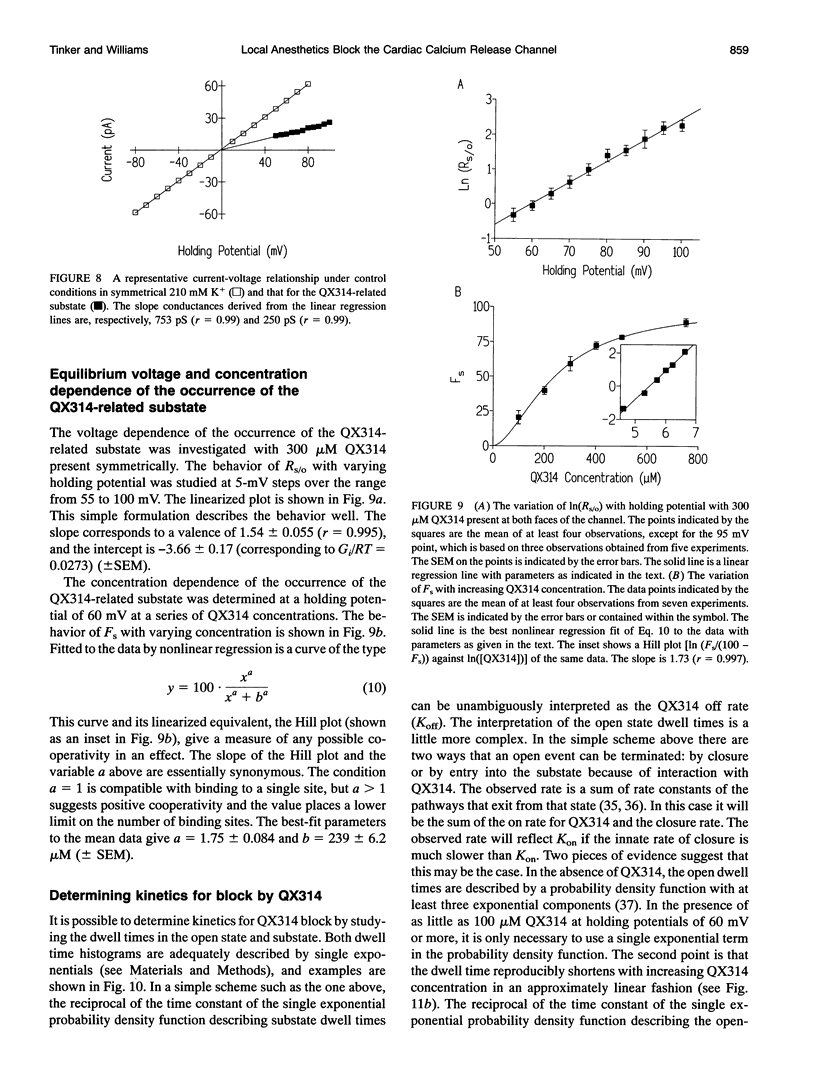
We have examined the effect of the charged local anesthetics QX314, QX222, and Procaine on monovalent cation conduction in the Ca2+ release channel of the sheep cardiac sarcoplasmic reticulum. All three blockers only affect cation conductance when present at the cytoplasmic face of the channel. QX222 and Procaine act as voltage-dependent blockers. With 500 Hz filtering, this is manifest as a relatively smooth reduction in single-channel current amplitude most prominent at positive holding potentials. Quantitative analysis gives an effective valence of approximately 0.9 for both ions and Kb(0)s of 9.2 and 15.8 mM for QX222 and Procaine, respectively. Analysis of the concentration dependence of block suggests that QX222 is binding to a single site with a Km of 491 microM at a holding potential of 60 mV. The use of amplitude distribution analysis, with the data filtered at 1 to 2 kHz, reveals that the voltage and concentration dependence of QX222 block occurs largely because of changes in the blocker on rate. The addition of QX314 has a different effect, leading to the production of a substate with an amplitude of approximately one-third that of the control. The substate's occurrence is dependent on holding potential and QX314 concentration. Quantitative analysis reveals that the effect is highly voltage dependent, with a valence of approximately 1.5 caused by approximately equal changes in the on and off rates. Kinetic analysis of the concentration dependence of the substate occurrence reveals positive cooperativity with at least two QX314s binding to the conduction pathway, and this is largely accounted for by changes in the on rate. A paradoxical increase in the off rate at high positive holding potentials and with increasing QX314 concentration at 80 mV suggests the existence of a further QX314-dependent reaction that is both voltage and concentration dependent. The substate block is interpreted physically as a form of partial occlusion in the vestibule of the conduction pathway giving a reduction in single-channel current by electrostatic means.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. H., Williams A. J. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J Gen Physiol. 1990 May;95(5):981–1005. doi: 10.1085/jgp.95.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain B. K., Volpe P., Fleischer S. Calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. General characteristics. J Biol Chem. 1984 Jun 25;259(12):7540–7546. [PubMed] [Google Scholar]
- Chamberlain B. K., Volpe P., Fleischer S. Inhibition of calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. J Biol Chem. 1984 Jun 25;259(12):7547–7553. [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Fox J. A. Examination of subconductance levels arising from a single ion channel. J Theor Biol. 1991 Dec 7;153(3):401–423. doi: 10.1016/s0022-5193(05)80578-8. [DOI] [PubMed] [Google Scholar]
- Dani J. A. Ion-channel entrances influence permeation. Net charge, size, shape, and binding considerations. Biophys J. 1986 Mar;49(3):607–618. doi: 10.1016/S0006-3495(86)83688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Appraisal of the physiological relevance of two hypothesis for the mechanism of calcium release from the mammalian cardiac sarcoplasmic reticulum: calcium-induced release versus charge-coupled release. Mol Cell Biochem. 1989 Sep 7;89(2):135–140. doi: 10.1007/BF00220765. [DOI] [PubMed] [Google Scholar]
- Hille B. The pH-dependent rate of action of local anesthetics on the node of Ranvier. J Gen Physiol. 1977 Apr;69(4):475–496. doi: 10.1085/jgp.69.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg S. R., Williams A. J. Single channel recordings from human cardiac sarcoplasmic reticulum. Circ Res. 1989 Nov;65(5):1445–1449. doi: 10.1161/01.res.65.5.1445. [DOI] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987 Nov 15;262(32):15637–15642. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- January C. T., Fozzard H. A. Delayed afterdepolarizations in heart muscle: mechanisms and relevance. Pharmacol Rev. 1988 Sep;40(3):219–227. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lindsay A. R., Manning S. D., Williams A. J. Monovalent cation conductance in the ryanodine receptor-channel of sheep cardiac muscle sarcoplasmic reticulum. J Physiol. 1991 Aug;439:463–480. doi: 10.1113/jphysiol.1991.sp018676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay A. R., Williams A. J. Functional characterisation of the ryanodine receptor purified from sheep cardiac muscle sarcoplasmic reticulum. Biochim Biophys Acta. 1991 Apr 26;1064(1):89–102. doi: 10.1016/0005-2736(91)90415-5. [DOI] [PubMed] [Google Scholar]
- Lucchesi K. J., Moczydlowski E. On the interaction of bovine pancreatic trypsin inhibitor with maxi Ca(2+)-activated K+ channels. A model system for analysis of peptide-induced subconductance states. J Gen Physiol. 1991 Jun;97(6):1295–1319. doi: 10.1085/jgp.97.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J Gen Physiol. 1982 May;79(5):869–891. doi: 10.1085/jgp.79.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Racker E. Mechanism of calcium release from skeletal sarcoplasmic reticulum. J Membr Biol. 1982;66(3):193–201. doi: 10.1007/BF01868494. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Moss G. W., Lucchesi K. J. Bovine pancreatic trypsin inhibitor as a probe of large conductance Ca(2+)-activated K+ channels at an internal site of interaction. Biochem Pharmacol. 1992 Jan 9;43(1):21–28. doi: 10.1016/0006-2952(92)90656-4. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D., Prod'hom B., Hess P. Interactions of protons with single open L-type calcium channels. pH dependence of proton-induced current fluctuations with Cs+, K+, and Na+ as permeant ions. J Gen Physiol. 1989 Jul;94(1):1–21. doi: 10.1085/jgp.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B., Stanfield P. R. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol. 1988 Nov;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran A., Schild L., Moczydlowski E. Divalent cation selectivity for external block of voltage-dependent Na+ channels prolonged by batrachotoxin. Zn2+ induces discrete substates in cardiac Na+ channels. J Gen Physiol. 1991 Jan;97(1):89–115. doi: 10.1085/jgp.97.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Henderson J. S., Meissner G. Single channel and 45Ca2+ flux measurements of the cardiac sarcoplasmic reticulum calcium channel. Biophys J. 1986 Nov;50(5):1009–1014. doi: 10.1016/S0006-3495(86)83543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Ma J. J., González A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. J Muscle Res Cell Motil. 1991 Apr;12(2):127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- Schild L., Ravindran A., Moczydlowski E. Zn2(+)-induced subconductance events in cardiac Na+ channels prolonged by batrachotoxin. Current-voltage behavior and single-channel kinetics. J Gen Physiol. 1991 Jan;97(1):117–142. doi: 10.1085/jgp.97.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol. 1986 Nov;88(5):573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A., Lindsay A. R., Williams A. J. A model for ionic conduction in the ryanodine receptor channel of sheep cardiac muscle sarcoplasmic reticulum. J Gen Physiol. 1992 Sep;100(3):495–517. doi: 10.1085/jgp.100.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A., Lindsay A. R., Williams A. J. Block of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by tetra-alkyl ammonium cations. J Membr Biol. 1992 Apr;127(2):149–159. doi: 10.1007/BF00233287. [DOI] [PubMed] [Google Scholar]
- Tinker A., Lindsay A. R., Williams A. J. Large tetraalkyl ammonium cations produce a reduced conductance state in the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel. Biophys J. 1992 May;61(5):1122–1132. doi: 10.1016/S0006-3495(92)81922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A., Williams A. J. Divalent cation conduction in the ryanodine receptor channel of sheep cardiac muscle sarcoplasmic reticulum. J Gen Physiol. 1992 Sep;100(3):479–493. doi: 10.1085/jgp.100.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annu Rev Physiol. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]
- Williams A. J. Ion conduction and discrimination in the sarcoplasmic reticulum ryanodine receptor/calcium-release channel. J Muscle Res Cell Motil. 1992 Feb;13(1):7–26. doi: 10.1007/BF01738423. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Jones R., Meissner G. Effects of local anesthetics on single channel behavior of skeletal muscle calcium release channel. J Gen Physiol. 1993 Feb;101(2):207–233. doi: 10.1085/jgp.101.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]


