Abstract
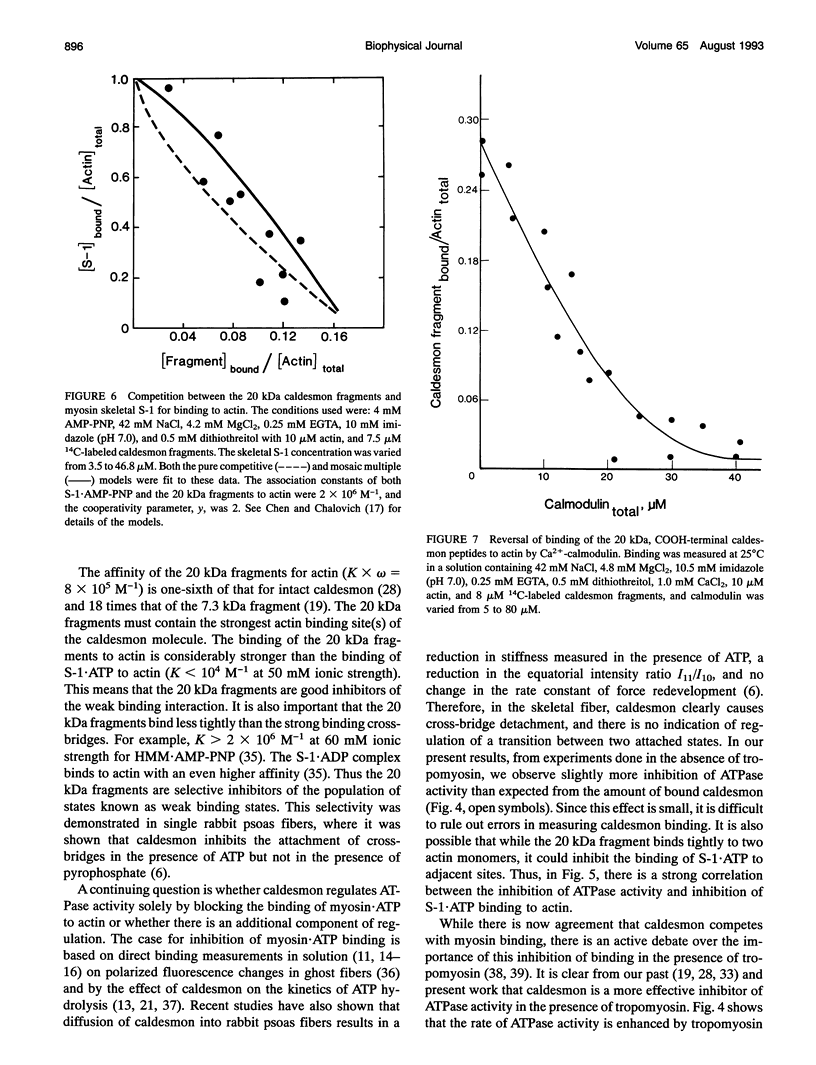
The protein caldesmon inhibits actin-activated ATP hydrolysis of myosin and inhibits the binding of myosin.ATP to actin. A fragment isolated from a chymotryptic digest of caldesmon contains features of the intact molecule that make it useful as a selective inhibitor of the binding of myosin.ATP complexes to actin without having the complexity of binding to myosin. The COOH-terminal 20 kDa region of caldesmon binds to actin with one-sixth the affinity of caldesmon with a stoichiometry of binding of one fragment per two actin monomers. This contrasts with the 1:6-9 stoichiometry of intact caldesmon. The binding of the 20 kDa fragments to actin is totally reversed by Ca(2+)-calmodulin and, like intact caldesmon, the 20 kDa fragments are competitive with the binding of myosin subfragments to actin. This competition with myosin binding is largely responsible for the inhibition of ATP hydrolysis, although both the fragments and intact caldesmon also reverse the potentiation of ATPase activity caused by tropomyosin. These polypeptides are useful both in defining the function of caldesmon and in studying the role of weakly bound cross-bridges in muscle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S., DasGupta G., Chalovich J. M., Reisler E. Immunochemical evidence for the binding of caldesmon to the NH2-terminal segment of actin. J Biol Chem. 1990 Nov 15;265(32):19652–19657. [PubMed] [Google Scholar]
- Bartegi A., Fattoum A., Derancourt J., Kassab R. Characterization of the carboxyl-terminal 10-kDa cyanogen bromide fragment of caldesmon as an actin-calmodulin-binding region. J Biol Chem. 1990 Sep 5;265(25):15231–15238. [PubMed] [Google Scholar]
- Bartegi A., Fattoum A., Kassab R. Cross-linking of smooth muscle caldesmon to the NH2-terminal region of skeletal F-actin. J Biol Chem. 1990 Feb 5;265(4):2231–2237. [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Chalovich J. M. Parallel inhibition of active force and relaxed fiber stiffness in skeletal muscle by caldesmon: implications for the pathway to force generation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5739–5743. doi: 10.1073/pnas.88.13.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Chalovich J. M., Bryan J., Benson C. E., Velaz L. Localization and characterization of a 7.3-kDa region of caldesmon which reversibly inhibits actomyosin ATPase activity. J Biol Chem. 1992 Aug 15;267(23):16644–16650. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M. Caldesmon and thin-filament regulation of muscle contraction. Cell Biophys. 1988 Jan-Jun;12:73–85. doi: 10.1007/BF02918351. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M., Chock P. B., Eisenberg E. Mechanism of action of troponin . tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981 Jan 25;256(2):575–578. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Cornelius P., Benson C. E. Caldesmon inhibits skeletal actomyosin subfragment-1 ATPase activity and the binding of myosin subfragment-1 to actin. J Biol Chem. 1987 Apr 25;262(12):5711–5716. [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Hemric M. E., Velaz L. Regulation of ATP hydrolysis by caldesmon. A novel change in the interaction of myosin with actin. Ann N Y Acad Sci. 1990;599:85–99. doi: 10.1111/j.1749-6632.1990.tb42367.x. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M., Yu L. C., Brenner B. Involvement of weak binding crossbridges in force production in muscle. J Muscle Res Cell Motil. 1991 Dec;12(6):503–506. doi: 10.1007/BF01738438. [DOI] [PubMed] [Google Scholar]
- Chen Y. D., Chalovich J. M. A mosaic multiple-binding model for the binding of caldesmon and myosin subfragment-1 to actin. Biophys J. 1992 Oct;63(4):1063–1070. doi: 10.1016/S0006-3495(92)81687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Ozawa J., Ogoma Y., Kondo Y. Interaction between chicken gizzard caldesmon and tropomyosin. J Biochem. 1988 Nov;104(5):734–737. doi: 10.1093/oxfordjournals.jbchem.a122542. [DOI] [PubMed] [Google Scholar]
- Graceffa P. Evidence for interaction between smooth muscle tropomyosin and caldesmon. FEBS Lett. 1987 Jun 22;218(1):139–142. doi: 10.1016/0014-5793(87)81034-7. [DOI] [PubMed] [Google Scholar]
- Greene L. E. Comparison of the binding of heavy meromyosin and myosin subfragment 1 in F-actin. Biochemistry. 1981 Apr 14;20(8):2120–2126. doi: 10.1021/bi00511a008. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Yamada S., Kanda K., Kimizuka F., Kato I., Sobue K. 35 kDa fragment of h-caldesmon conserves two consensus sequences of the tropomyosin-binding domain in troponin T. Biochem Biophys Res Commun. 1989 May 30;161(1):38–45. doi: 10.1016/0006-291x(89)91556-8. [DOI] [PubMed] [Google Scholar]
- Hegmann T. E., Schulte D. L., Lin J. L., Lin J. J. Inhibition of intracellular granule movement by microinjection of monoclonal antibodies against caldesmon. Cell Motil Cytoskeleton. 1991;20(2):109–120. doi: 10.1002/cm.970200204. [DOI] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Characterization of caldesmon binding to myosin. J Biol Chem. 1990 Nov 15;265(32):19672–19678. [PMC free article] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Effect of caldesmon on the ATPase activity and the binding of smooth and skeletal myosin subfragments to actin. J Biol Chem. 1988 Feb 5;263(4):1878–1885. [PubMed] [Google Scholar]
- Horiuchi K. Y., Chacko S. Caldesmon inhibits the cooperative turning-on of the smooth muscle heavy meromyosin by tropomyosin-actin. Biochemistry. 1989 Nov 14;28(23):9111–9116. doi: 10.1021/bi00449a023. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Samuel M., Chacko S. Mechanism for the inhibition of acto-heavy meromyosin ATPase by the actin/calmodulin binding domain of caldesmon. Biochemistry. 1991 Jan 22;30(3):712–717. doi: 10.1021/bi00217a019. [DOI] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- Katsuyama H., Wang C. L., Morgan K. G. Regulation of vascular smooth muscle tone by caldesmon. J Biol Chem. 1992 Jul 25;267(21):14555–14558. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leszyk J., Mornet D., Audemard E., Collins J. H. Amino acid sequence of a 15 kilodalton actin-binding fragment of turkey gizzard caldesmon: similarity with dystrophin, tropomyosin and the tropomyosin-binding region of troponin T. Biochem Biophys Res Commun. 1989 Apr 14;160(1):210–216. doi: 10.1016/0006-291x(89)91642-2. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985 Nov 1;231(3):517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. Inhibition of actin-tropomyosin activation of myosin MgATPase activity by the smooth muscle regulatory protein caldesmon. J Biol Chem. 1992 Aug 25;267(24):16796–16800. [PubMed] [Google Scholar]
- Marston S. B., Smith C. W. Purification and properties of Ca2+-regulated thin filaments and F-actin from sheep aorta smooth muscle. J Muscle Res Cell Motil. 1984 Oct;5(5):559–575. doi: 10.1007/BF00713261. [DOI] [PubMed] [Google Scholar]
- Marston S. Aorta caldesmon inhibits actin activation of thiophosphorylated heavy meromyosin Mg2+-ATPase activity by slowing the rate of product release. FEBS Lett. 1988 Sep 26;238(1):147–150. doi: 10.1016/0014-5793(88)80245-x. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Nowak E., Borovikov Y. S., Dabrowska R. Caldesmon weakens the bonding between myosin heads and actin in ghost fibers. Biochim Biophys Acta. 1989 Dec 21;999(3):289–292. doi: 10.1016/0167-4838(89)90011-3. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Zeugner C., Troschka M., Chalovich J. M. Caldesmon and a 20-kDa actin-binding fragment of caldesmon inhibit tension development in skinned gizzard muscle fiber bundles. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5904–5908. doi: 10.1073/pnas.90.13.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Velaz L., Hemric M. E., Benson C. E., Chalovich J. M. The binding of caldesmon to actin and its effect on the ATPase activity of soluble myosin subfragments in the presence and absence of tropomyosin. J Biol Chem. 1989 Jun 5;264(16):9602–9610. [PubMed] [Google Scholar]
- Velaz L., Ingraham R. H., Chalovich J. M. Dissociation of the effect of caldesmon on the ATPase activity and on the binding of smooth heavy meromyosin to actin by partial digestion of caldesmon. J Biol Chem. 1990 Feb 15;265(5):2929–2934. [PubMed] [Google Scholar]
- Walker G., Kerrick W. G., Bourguignon L. Y. The role of caldesmon in the regulation of receptor capping in mouse T-lymphoma cell. J Biol Chem. 1989 Jan 5;264(1):496–500. [PubMed] [Google Scholar]
- Watson M. H., Kuhn A. E., Mak A. S. Caldesmon, calmodulin and tropomyosin interactions. Biochim Biophys Acta. 1990 Aug 13;1054(1):103–113. doi: 10.1016/0167-4889(90)90211-u. [DOI] [PubMed] [Google Scholar]
- Watson M. H., Kuhn A. E., Novy R. E., Lin J. J., Mak A. S. Caldesmon-binding sites on tropomyosin. J Biol Chem. 1990 Nov 5;265(31):18860–18866. [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Yamashiro S., Matsumura F. Mitosis-specific phosphorylation of caldesmon: possible molecular mechanism of cell rounding during mitosis. Bioessays. 1991 Nov;13(11):563–568. doi: 10.1002/bies.950131103. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Sakuma M., Yagi K. Calmodulins from muscles of marine invertebrates, scallop and sea anemone. J Biochem. 1980 May;87(5):1313–1320. doi: 10.1093/oxfordjournals.jbchem.a132869. [DOI] [PubMed] [Google Scholar]