Abstract
Neuropilins (NP1 and NP2) are vascular endothelial growth factor (VEGF) receptors that mediate developmental and tumor angiogenesis. Transgenic mice, in which both NP1 and NP2 were targeted (NP1−/−NP2−/−) died in utero at E8.5. Their yolk sacs were totally avascular. Mice deficient for NP2 but heterozygous for NP1 (NP1+/−NP2−/−) or deficient for NP1 but heterozygous for NP2 (NP1−/−NP2+/−) were also embryonic lethal and survived to E10–E10.5. The E10 yolk sacs and embryos were easier to analyze for vascular phenotype than the fragile poorly formed 8.5 embryos. The vascular phenotypes of these E10 mice were very abnormal. The yolk sacs, although of normal size, lacked the larger collecting vessels and had less dense capillary networks. PECAM staining of yolk sac endothelial cells showed the absence of branching arteries and veins, the absence of a capillary bed, and the presence of large avascular spaces between the blood vessels. The embryos displayed blood vessels heterogeneous in size, large avascular regions in the head and trunk, and blood vessel sprouts that were unconnected. The embryos were about 50% the length of wild-type mice and had multiple hemorrhages. These double NP1/NP2 knockout mice had a more severe abnormal vascular phenotype than either NP1 or NP2 single knockouts. Their abnormal vascular phenotype resembled those of VEGF and VEGFR-2 knockouts. These results suggest that NRPs are early genes in embryonic vessel development and that both NP1 and NP2 are required.
Keywords: vascular endothelial growth factor‖vascular endothelial growth factor receptors‖blood vessels‖endothelial cells‖semaphorins
Vascular endothelial growth factor (VEGF) is a well characterized angiogenesis factor. It induces the migration, proliferation, and survival of endothelial cells (EC) (1) and mediates early developmental vasculogenesis and angiogenesis (2). VEGF knockouts, even heterozygotes, are embryonic lethal, indicating that VEGF vascular activity is tightly concentration-dependent (3). A vascular role for VEGF has been implicated in collateral vessel formation, wound healing, and in the response to ischemia (4). VEGF is also a major contributor to tumor angiogenesis. Tumor cells produce high levels of VEGF (5), which stimulate tumor vascularization in response to hypoxia. VEGF antagonists such as anti-VEGF antibodies and soluble VEGF receptors inhibit tumor vascularization and significantly repress tumor growth (6, 7). VEGF activities are mediated by two high affinity receptor tyrosine kinases Flt-1 (VEGFR-1) and KDR/Flk-1 (VEGFR-2) (8, 9). VEGFR-2 knockouts result in impairment of early stages of vasculogenesis and angiogenesis, whereas VEGFR-1 knockouts affect later stages of angiogenesis such as tube formation (10, 11).
Recently, we identified another VEGF receptor, neuropilin-1 (NP1), which is expressed by EC and tumor cells (12, 13). NP1 was first described as a cell-surface glycoprotein expressed on axons (14, 15) and shown to be a receptor for the semaphorin/collapsin family of neuronal guidance mediators. VEGF165 binds to NP1 with a Kd of 2 × 10−10 M, as does semaphorin 3A (Sema3A) (16). Other members of the VEGF family, VEGF-B and placental growth factor, are also ligands for NP1 (17, 18). Coexpression of NP1 and KDR in porcine aortic EC enhanced VEGF165 binding to KDR and the KDR-mediated chemotactic activity of VEGF165, suggesting that NP1 is a coreceptor for VEGFR-2 (13). On the other hand, in neurons NP1 seems to be a coreceptor for plexins, which are transmembrane signal-transducing receptors (19).
There are two neuropilins, NP1 and NP2, and they localize to different chromosomes. They have similar domain structures and a 45–50% amino acid sequence homology (20). There is ample evidence that NPs play a role in angiogenesis. Both NP1 and NP2 are expressed by EC and bind VEGF165 to these cells (21). Overexpression of NP1 in transgenic mice resulted in excess capillary formation, dilated blood vessels, and extensive hemorrhage (22). NP1−/− mice die at E12.5–E13.5. Besides severe abnormalities in the trajectories of the cranial and spinal efferent fibers that express NP1, there were some vascular defects as well, including partial impairment of neural vascularization, improper development of brachial arch arteries and great vessels, and disorganized vascular networks in the yolk sac (23). On the other hand, no discernable abnormal vascular phenotype has been reported for NP2-deficient mice (24, 25). Mice engineered to express only VEGF120 have fewer coronary vessels and a 4-fold reduction in capillary density of the heart, one possible explanation being that VEGF120 alone is insufficient for normal angiogenesis because it cannot bind to NP1 (26). NP1 also has a role in tumor angiogenesis. Conditional overexpression of NP1 in prostate carcinoma cells resulted in enhanced tumor angiogenesis and growth characterized by high microvessel density, dilated blood vessels, increased proliferating EC, and notably less tumor cell and EC apoptosis, compared with noninduced controls (27), consistent with increased angiogenesis correlating with decreased apoptosis (28).
NP1 and NP2 have some overlapping properties such as expression by neurons, EC, and tumor cells. However, they differ as well, for example, in the differential activation of NPs by their ligands. Sema3A activates NP1, whereas Sem3F activates NP2 (20, 29). VEGF165 binds NP1 and NP2 whereas VEGF145 binds only NP2 (30). These examples of differential properties suggest that targeting both NP1 and NP2 genes together might yield a more severe abnormal phenotype than targeting just one of the two genes. Therefore, we generated transgenic mice in which both NP1 and NP2 were targeted. We report here that NP1−/−NP2−/− mice died very early in utero at E8.5 and exhibited early defects in blood vessel development in the yolk sacs and in the embryos. The double NP1/NP2 knockout mice had a more severe abnormal vascular phenotype than either NP1 or NP2 single knockouts. Interestingly, mice that were deficient for NP2 but heterozygous for NP1 (NP1+/−NP2−/−) or deficient for NP1 but heterozygous for NP2 (NP1−/−NP2+/−) were also embryonic lethal but survived to E10–E10.5. These mice were characterized by greatly diminished yolk sac vasculature, disorganized blood vessels, and growth-retarded embryos. Together, these results suggest that both NP1 and NP2 are required for normal vasculogenesis and angiogenesis in early embryonic development.
Materials and Methods
Northern Blot Analysis.
Northern blot was performed as previously described (13). Briefly, E9.5 whole embryos were excised and quickly minced in RNAzol (Tel-Test, Friendswood, TX). Total RNA was isolated and electrophoresed on 1% formaldehyde-agarose gels and transferred onto nylon membranes (Bio-Rad). The membranes were hybridized with 32P-labeled fragments of mouse cDNA corresponding to nucleotides 12–530 of the NP1 ORF and nucleotides 5–620 of the NP2 ORF. A BAS photoimaging system (Fuji) was used for detection.
Genotyping.
Germ-line transmission of the targeted NP2 locus in heterozygous mice was confirmed by Southern blotting by using the probe shown in Fig. 1A. Genotyping was carried out by genomic PCR of crude DNA samples prepared from a small piece of yolk sac. Primers for Neo are 5′-GGCCTCTTCGCTATTACG-3′ and 5′-GAGACTGGCCAAGCGGGTGTAAC-3′. Primers for the replaced intronic fragment are 5′-TGGCTTCTCTCCATTAGCTGTCG-3′ and 5′-GAGACTGGCCAAGCGGGTGTAAC-3′. NP1 genotyping has been previously described (15). The embryos that were genotyped included 50 embryos at E8.0, 76 embryos at E9.5, 60 embryos at E12.0, and 54 embryos at birth.
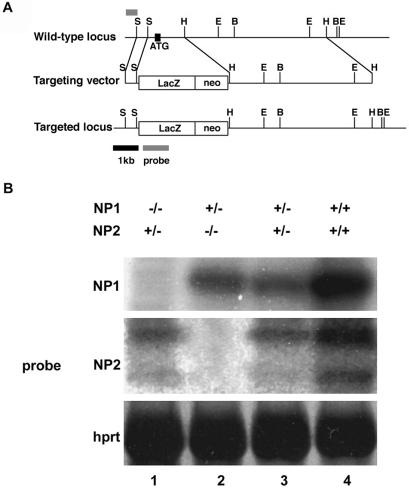
Figure 1.
NP1 and NP2 knockouts. (A) Targeting vector for disruption of the NP2 gene in mouse embryonic stem cells. (Top) Restriction map of a mouse NP2 genomic fragment showing sites for the restriction enzymes EcoRI (E), BamHI (B), HindIII (H), and SmaI (S). The small bar depicts the region used as a probe for Southern blot analysis. (Middle) Targeting vector in which a region encompassing the first coding exon and the proximal part of the next intron of NP2 (1.7 kb) are replaced by a lacZ-neo cassette (4 kb). (Bottom) Predicted structure of the targeted NP2 allele. (B) Northern blot analysis of NP1 and NP2 mRNA expression levels at E9.5 for various NP1 and NP2 genotypes. Genotyping was carried out on littermates. Lane 1, NP1−/−NP2+/−; lane 2, NP1+/−NP2−/−; lane 3, NP1+/−NP2+/−; lane 4, NP1+/+NP2+/+. In heterozygotes, NP mRNA levels are less than one-half of wild-type mRNA levels. Densitometer readings: NP1 Northern blot (Top), densities in lanes 1–4 are 0, 350, 250, and 1,050 arbitrary units, respectively. NP2 Northern blot (Middle, upper band) densities in lanes 1–4 are 200, 0, 180, and 770 arbitrary units, respectively.
PECAM-1 Immunostaining.
Embryos and yolk sacs were removed and fixed in cold 2% paraformaldehyde/20 mM sodium phosphate, pH 7.4, for 30 min. For PECAM staining, yolk sacs and whole-mount embryos were incubated with anti-PECAM-1 antibody (clone MEC13.3, PharMingen) and then with alkaline phosphatase-conjugated secondary antibody (Promega) as previously described (11).
Results
Production of NP1 and NP2 Single and Double Knockouts.
VEGF promotes the proliferation, migration, and survival of EC (1). VEGF165 crosslinking and Northern blot analysis demonstrated that human umbilical vein endothelial cells expressed VEGFR-2, NP1, and NP2 but neither VEGFR-1 nor VEGFR-3 (not shown). Since both NP1 and NP2 are expressed by EC as receptors for VEGF165, it was deemed necessary to target both to determine accurately the function of NPs in blood vessel development.
To compare vascular phenotypes of NP1−/− and NP2−/− knockouts, heterozygous mice obtained from Dr. H. Fujisawa (15) were mated to produce NP1-deficient (NP1−/−) mice. To generate NP2−/− mice, a targeting vector was constructed in which the translated portion of the first coding exon and the proximal part of the next intron of NP2 were replaced with a promoterless Escherichia coli β-galactosidase gene to produce NP2+/lacZ mice (Fig. 1A). These heterozygote mice (effectively NP2+/−) were mated to produce NP2−/− mice. Balb/C mice were mated five times to determine genetic background.
Northern blot analysis indicated that NP1−/− and NP2−/− mice did not express NP1 (Fig. 1B Top, lane 1) or NP2 (Fig. 1B Middle, lane 2), respectively. The lack of NP1 expression was not compensated by NP2 overexpression (Fig. 1B Middle, lane 1 compared with lane 3) nor was lack of NP2 expression compensated by NP1 overexpression (Fig. 1B Top, lane 2 compared with lane 3). The NP1−/− genotype was embryonic lethal, and the mice died at E12.5. They had vascular defects as previously reported (23), for example, transposition of great vessels. On the other hand, NP2−/− mice were viable, surviving up to 2 weeks after birth and with no apparent abnormal vascular phenotype, which confirmed previous results (24, 25). NP1+/− heterozygotes were mated with NP2+/− heterozygotes. F1 mice were born in a normal Mendelian ratio. Of the F1 mice, NP1+/−NP2+/− male and female mice were mated to generate double knockout NP1−/−NP2−/− mice.
Vasculature Defects in the Yolk Sac.
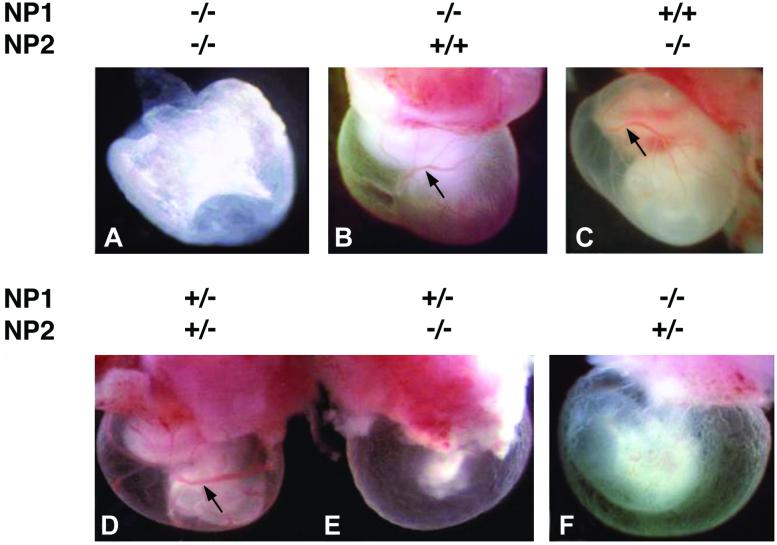
NP1−/−NP2−/− mice died at about E8.5. No organized vessels were detected in the yolk sac (Fig. 2A), and the embryos within the yolk sac were almost completely resorbed. By contrast, in mice deficient only for NP1, mostly normal blood vessel network formation occurred both in the yolk sac (Fig. 2B) and embryo (not shown) at E10.0. Normal yolk sac vasculature was observed in mice deficient for NP2 only (Fig. 2C). Thus, the abnormal vascular phenotype in the yolk sac of the double NP knockout was more severe than either NP knockout alone.
Figure 2.
Impairment of yolk sac blood vessel formation in double NP knockout mouse embryos. (A) Yolk sac of E8.5 NP1−/−NP2−/− double knockout mouse. No blood vessels can be detected. (B and C) E10 yolk sacs of NP1−/−NP2+/+ and NP1+/+NP2−/− mice, respectively. Note presence of dense capillary plexus and large collection vessels (black arrow). (D) E10 yolk sac of NP1+/−NP2+/− control mouse. Note presence of dense capillary plexus with large collection vessels (black arrow). (E) E10 yolk sac of NP1+/−NP2−/− mouse embryo. Larger collecting vessels seem to be absent, and capillary density is very diminished. (F) E10 yolk sac of NP1−/−NP2+/− mouse. Blood vessel impairment is similar to that in E. The embryos in D and E (shown together) and F are littermates.
Interestingly, mice deficient for NP1 or NP2 but heterozygous for the other NP gene died in utero but survived longer, to E10–E10.5, making analysis of the embryo phenotype much more feasible then analysis of E8.5 NP1−/−NP2−/− embryos, which were poorly formed and very fragile. The yolk sacs of NP1+/−NP2+/− mice at E10.0 had the same phenotype as wild-type mice and served as controls (Fig. 2D). These normal yolk sacs had a dense capillary plexus, large collection vessels, and the embryo within the yolk sac was readily visible. On the other hand, mice deficient for NP2 but heterozygous for NP1 (Fig. 2E) and mice deficient for NP1 but heterozygous for NP2 (Fig. 2F) had yolk sacs of normal size but lacked the larger collecting vessels and contained irregular and much less dense capillary networks.
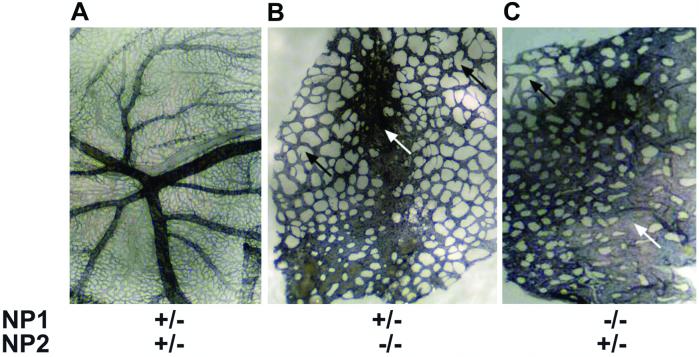
The embryos in Fig. 2 D–F, which were littermates, had their yolk sacs and embryos collected and fixed separately. To analyze vascular network formation, yolk sacs were stained with antibodies to the specific EC marker PECAM. The yolk sacs of NP1+/−NP2+/− embryos had branching arteries and veins as well as a fine capillary bed (Fig. 3A). By contrast, arterial and venous branching was not observed in the yolk sacs of NP1+/−NP2−/− (Fig. 3B) or NP−/−NP2+/− (Fig. 3C) mice. Instead, the vasculature was characterized by vessel heterogeneity, disorganization, and immaturity. Thickened blood vessels, lack of capillaries, and large avascular spaces between the blood vessels, perhaps caused by the absence of capillaries, were observed. There were also occasional dead-ended sprouts not connected to other sprouts.
Figure 3.
PECAM staining of E10 yolk sacs shown in Fig. 2. Blood vessel yolk sacs were stained with the EC specific marker PECAM. The yolk sacs in A–C correspond to the yolk sacs in Fig. 2 D–F, respectively. (A) The NP1+/−NP2+/− yolk sac has large branching vessels and a dense capillary network. (B) The NP1+/−NP2−/− yolk sac lacks large branching vessels and lacks a capillary network. Some blood vessels are fused (white arrow). Large avascular spaces are found between the blood vessels (black arrows). (C) NP1−/−NP2+/− yolk sac. Blood vessel impairment is severe with lack of large branching vessels and capillaries, and with fused vessels (white arrow) and avascular regions (black arrow).
Vasculature Defects in the Embryo.
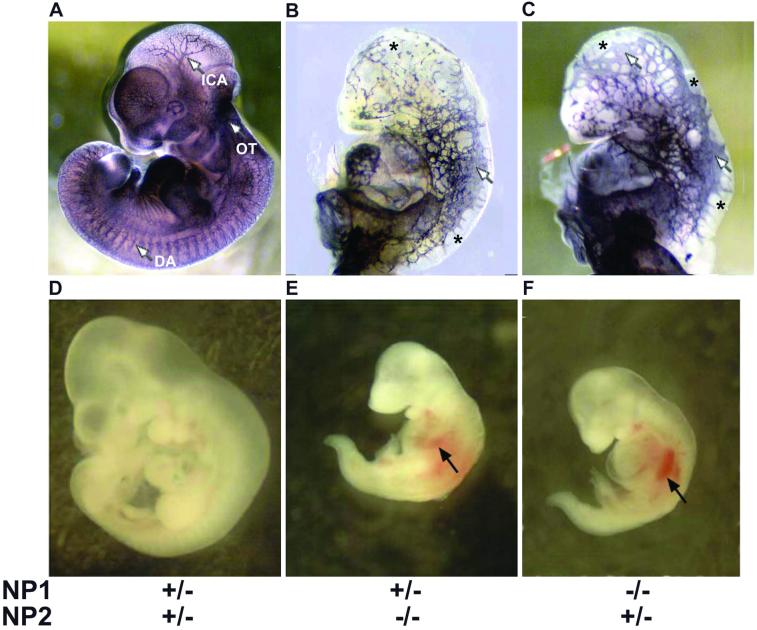
The embryos inside the poorly vascularized yolk sacs also showed severe vascular impairment. The control E10 embryo (NP1+/−NP2+/−) had well organized blood vessels as determined by PECAM staining (Fig. 4A). For example, large branching intracranial arteries, the dorsal aorta, and the outflow tract were detected. Small capillary networks were detected in the head and distal part of the body. In contrast, the NP1+/−NP2−/− embryo (Fig. 4B) had severe vascular abnormalities. Blood vessels were disorganized, heterogeneous in size, and in some cases thickened. Blood vessel density was low in the head region, and there were avascular regions in both the head and trunk. The dorsal aorta was poorly formed, and the outflow tract was undetectable. There was some small vessel sprouting, but unconnected to other sprouts and without formation of a capillary plexus. Similar results including avascular regions, blood vessel size heterogeneity, thickened blood vessels, and abnormal formation of the dorsal aorta and outflow tract were observed in NP1−/−NP2+/− embryos (Fig. 4C). However, the blood vessel density in the head region (Fig. 4C) appeared to be higher than in Fig. 4B.
Figure 4.
Whole mount embryos with and without PECAM staining. The embryos in panels A–C and D–F are the same embryos shown within the yolk sacs in Fig. 2 D–F, respectively. (A–C) PECAM staining. The embryos in B and C were enlarged to the size of the embryo in A for easier comparison. (A) E10 NP1+/−NP2+/− control embryo. The blood vessel network is dense and reaches to the distal part of the embryo. ICA, intracranial artery; OF, outflow tract; DA, dorsal aorta. (B) E10 NP1+/−NP2−/− embryo. Blood vessels in the head are sparse. There are avascular regions in the head and body (asterisks). There are fused large vessels (white arrow) and small sprouting vessels that do not reach the distal regions. The outflow tract and dorsal aorta are not readily detectable. (C) E10 NP1−/−NP2+/− embryo. The vascular phenotype is similar to B, although blood vessel density in the head seems to be greater. There are avascular regions (asterisks), fused vessels (white arrows), and nonconnected sprouts near the peripheries. Large avascular regions are seen between the vessels. The outflow tract and dorsal aorta are not readily detectable. (D–F) Non-PECAM stained embryos corresponding to A–C, respectively, at their proper magnifications. (D) E10 NP1+/−NP2+/− control embryo. Normal development is evident. (E) E10 NP1+/−NP2−/− embryo and (F) E10 NP1−/−NP2+/− embryo. Both embryos are about 50% in length compared with the control littermate in D. To detect extravascular hemorrhage, the blood in the vasculature was washed out by perfusion of the heart with PBS. Multiple hemorrhages occurred in the both embryos (black arrows).
Whole mount (non-PECAM stained) embryos that were within the yolk sacs in Fig. 2 D–F are shown in Fig. 4 D–F, respectively. NP1+/−NP2−/− (Fig. 4E) and NP1−/−NP2+/− (Fig. 4F) embryos were much smaller than control littermates (Fig. 4D), about 50% less in length. In addition, multiple hemorrhaging occurred in both of these embryos (Fig. 4 E and F).
Taken together, analyses of embryos and their yolk sacs demonstrate that double NP knockouts display severe vascular defects in early embryogenesis.
Discussion
Our results suggest that targeting both NP1 and NP2 genes results in a severe impairment of embryonic blood vessel development. The double knockout abnormal vascular phenotype is more severe than that obtained by targeting either NP gene alone. Double NP knockouts (NP1−/−NP2−/−) survived only until E8.5 and were characterized by lack of blood vessel formation in the yolk sac, blood vessels abnormalities in the embryo, and impaired embryo development. On the other hand, NP1 or NP2 deficient mice, which survive to E12–E13 and to postnatal life, respectively, were comparable to wild-type mice in the density of yolk sac blood vessels, embryo size, and blood vessel organization.
It was difficult to analyze the embryos at E8.5 histologically because of their small size, fragility, and tendency to be resorbed. However, an abnormal vascular phenotype occurred even when one of the NPs was lacking but the other NP had one wild-type allele. These mice, NP1+/−NP2−/− and NP1−/−NP2+/−, survived until about E10.5, and at this stage the yolk sacs and embryos could be analyzed more readily. PECAM staining of yolk sacs demonstrated a striking lack of large collecting blood vessels and capillaries. The lack of capillaries might reflect a defect in branching, resulting from the inability of EC to migrate and proliferate because of diminished VEGF signaling. Many of the blood vessels were thickened, possibly arterial–venous malformations that resulted from direct artery–vein interactions in the absence of capillaries. There were large avascular regions between blood vessels.
PECAM analysis of whole mount knockout embryos showed regions of avascularity, for example, in the head and trunk regions. The intracranial arteries, dorsal aorta, and outflow tract were poorly formed. Blood vessel density was sparse, and there were large avascular spaces between the blood vessels, some of which were thickened. Small sprouts were seen but not connected to other sprouts. The embryos were relatively small, about 40–50% reduced in length compared with wild type, possibly as a result of severe anemia, hypoxia, or lack of nourishment because of an improperly vascularized yolk sac. It is not clear why having one wild-type copy of NP1 or NP2 when the other gene is totally absent enhances embryo viability by several days. It could be because of an NP dose effect in which the NP ligands, VEGF165 and/or semaphorins, can function partially when one NP gene copy is expressed. An adverse effect on angiogenesis with the loss of one gene but heterozygous in a related gene has been reported for the Id family (31). Id1−/−Id3−/− mice survived to day E10.5 and displayed vascular malformations of the brain. However, Id1−/−Id3−/+ mice were born but did not support tumor growth because of poor vascularization.
Lack of yolk sac vasculature in NP double knockouts at E8.5 is one of the earliest abnormal vascular phenotypes reported to date. The abnormal vascular phenotype of NP1−/−NP2−/− mice resembled that of VEGFR-2 deficient mice, which were embryonic lethal and died in utero at E8.5–E9.5 (10). In these mice, blood islands were absent, and embryonic and yolk sac blood vessels were not observed. The double NP knockout also resembles the vascular phenotype of VEGF-deficient mice, which even as heterozygotes died in utero at E9.5–E10.5 because of abnormalities in blood island development and angiogenesis (3). The abnormal vascular phenotype of NP double knockout mice manifested itself earlier than that of the EC receptors VEGFR-1 and Tie-2, which mediate later stages of blood vessel development, such as the ability of differentiated EC to assemble and form capillary-like tubes (11, 32).
Our results demonstrate that both NP1 and NP2 genes are functional in the vasculature and that both genes are needed to mediate normal vasculogenesis and angiogenesis in the developing yolk sac and embryo. These two receptors must provide some as yet undetermined nonoverlapping function in blood vessel formation so that both are needed. For example, NP1 is activated by Sema3A but not Sema3F, whereas NP2 is activated by Sema3F but not Sema3A (20, 29). Thus, double knockouts in theory would adversely affect both Sema3A and Sema3F activity, whereas Sema3A would still be active in an NP2 knockout and Sema3F in an NP1 knockout.
NPs are atypical in that they are specific high affinity receptors for two structurally disparate ligands, members of the VEGF family (13) and members of the semaphorin family (16, 29). NPs do not seem to be receptor tyrosine kinases but might act as adaptor proteins for receptor tyrosine kinases, VEGFR-2 in the case of VEGF (13), and plexins in the case of semaphorins (33). One question is whether the vascular phenotype of NP double knockout mice is due to impairment of VEGF interactions with NPs, semaphorin interactions with NPs, or both interactions. It is plausible that knocking out NPs affects VEGF activity as NP1 mediates KDR activity (13). In addition, the vascular phenotype of NP1−/−NP2−/− mice resembles that of VEGF and VEGFR-2 knockout mice. Semaphorins may also be affected by lack of NPs. Sema3A inhibited in vitro angiogenesis via NP1 (34). However, no vascular abnormalities were reported in sema3A-deficient mice (35, 36). Sema3A/NP interactions might mediate embryonic cardiovascular development. The cardiovascular anomalies in NP1−/− mice (23), such as aortic arch defects, were attributed to inappropriate migration or differentiation of cardiac neural crest cells (37). These cells express NP1, and their migration is regulated by Sema3A (38), which is strongly expressed in embryonic heart (36). It is plausible that NPs mediate both VEGF and semaphorin signal transduction pathways in vivo, with VEGF/NP interactions mediating angiogenesis and semaphorin/NP interactions mediating cardiovascular function.
In summary, the data presented here strongly suggest that both NP1 and NP2 are necessary for normal yolk sac and embryonic blood vessel development.
Acknowledgments
We thank Dr. H. Yoshida for whole mount embryo PECAM staining, Dr. R. Otani for animal feeding, and Dr. A. Ogai and T. Fukushima for technical assistance. We thank Eric Santiestevan for preparing the figures and Drs. Roni Mamluk, Gerhard Raab, Shay Soker (Children's Hospital, Boston), and Dr. Patricia D'Amore (Schepens Institute, Boston) for reading the manuscript. This study was supported by Grants-in-aid for Scientific Research Nos. 12470153 and 12877107 from the Ministry of Education, Science, Sports and Culture, Japan, and by grants from the Smoking Research Foundation in Japan (to S.T.) and National Cancer Institute Grants CA37392 and CA 45548 (to M.K.).
Abbreviations
- NP1
neuropilin-1
- NP2
neuropilin-2
- VEGF
vascular endothelial growth factor
- EC
endothelial cell
- SemaA
semaphorin A
References
- 1.Ferrara N, Davis-Smith T. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 2.Klagsbrun M, Soker S. Curr Biol. 1993;3:699–702. doi: 10.1016/0960-9822(93)90073-w. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea K S, Powell-Braxton L, Hillan K J, Moore M W. Nature (London) 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Curr Interv Cardiol Rep. 1999;1:322–335. [PubMed] [Google Scholar]
- 5.Dvorak H F, Sioussat T M, Brown L F, Berse B, Nagy J A, Sotrel A, Manseau E J, Van de Water L, Senger D R. J Exp Med. 1991;174:1275–1278. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 7.Kendall R L, Wang G, Thomas K A. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 8.Quinn T P, Peters K G, De Vries C, Ferrara N, Williams L T. Proc Natl Acad Sci USA. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terman B I, Dougher-Vermazen M, Carrion M E, Dimitrov D, Armellino D C, Gospodarowicz D, Bohlen P. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 10.Shalaby F, Rossant J, Yamaguchi T P, Gertsenstein M, Wu X F, Breitman M L, Schuh A C. Nature (London) 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 11.Fong G H, Rossant J, Gertsenstein M, Breitman M L. Nature (London) 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 12.Soker S, Fidder H, Neufeld G, Klagsbrun M. J Biol Chem. 1996;271:5761–5767. doi: 10.1074/jbc.271.10.5761. [DOI] [PubMed] [Google Scholar]
- 13.Soker S, Takashima S, Miao H Q, Neufeld G, Klagsbrun M. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 14.Satoda M, Takagi S, Ohta K, Hirata T, Fujisawa H. J Neurosci. 1995;15:942–955. doi: 10.1523/JNEUROSCI.15-01-00942.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 16.He Z, Tessier-Lavigne M. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 17.Makinen T, Olofsson B, Karpanen T, Hellman U, Soker S, Klagsbrun M, Eriksson U, Alitalo K. J Biol Chem. 1999;274:21217–21222. doi: 10.1074/jbc.274.30.21217. [DOI] [PubMed] [Google Scholar]
- 18.Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R, Baumann H, Neufeld G. J Biol Chem. 1998;273:22272–22278. doi: 10.1074/jbc.273.35.22272. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Strittmatter S M. Neuron. 2001;29:429–439. doi: 10.1016/s0896-6273(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 20.Giger R J, Urquhart E R, Gillespie S K, Levengood D V, Ginty D D, Kolodkin A L. Neuron. 1998;21:1079–1092. doi: 10.1016/s0896-6273(00)80625-x. [DOI] [PubMed] [Google Scholar]
- 21.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. J Biol Chem. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 22.Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Development (Cambridge, UK) 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. Development (Cambridge, UK) 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Bagri A, Zupicich J A, Zou Y, Stoeckli E, Pleasure S J, Lowenstein D H, Skarnes W C, Chedotal A, Tessier-Lavigne M. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- 25.Giger R J, Cloutier J F, Sahay A, Prinjha R K, Levengood D V, Moore S E, Pickering S, Simmons D, Rastan S, Walsh F S, et al. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P, Ng Y S, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar V V, Stalmans I, Mattot V, et al. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 27.Miao H Q, Lee P, Lin H, Soker S, Klagsbrun M. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 28.Holmgren L, O'Reilly M S, Folkman J. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 29.Kolodkin A L, Levengood D V, Rowe E G, Tai Y T, Giger R J, Ginty D D. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 30.Cohen T, Gluzman-Poltorak Z, Brodzky A, Meytal V, Sabo E, Misselevich I, Hassoun M, Boss J H, Resnick M, Shneyvas D, et al. Biochem Biophys Res Commun. 2001;284:395–403. doi: 10.1006/bbrc.2001.4958. [DOI] [PubMed] [Google Scholar]
- 31.Lyden D, Young A Z, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader B L, Hynes R O, Zhuang Y, Manova K, Benezra R. Nature (London) 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 32.Suri C, Jones P F, Patan S, Bartunkova S, Maisonpierre P C, Davis S, Sato T N, Yancopoulos G D. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 33.Winberg M L, Noordermeer J N, Tamagnone L, Comoglio P M, Spriggs M K, Tessier-Lavigne M, Goodman C S. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- 34.Miao H Q, Soker S, Feiner L, Alonso J L, Raper J A, Klagsbrun M. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Neuron. 1997;19:519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- 36.Behar O, Golden J A, Mashimo H, Schoen F J, Fishman M C. Nature (London) 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 37.Miyagawa-Tomita S, Waldo K, Tomita H, Kirby M L. Am J Anat. 1991;192:79–88. doi: 10.1002/aja.1001920109. [DOI] [PubMed] [Google Scholar]
- 38.Eickholt B J, Mackenzie S L, Graham A, Walsh F S, Doherty P. Development (Cambridge, UK) 1999;126:2181–2189. doi: 10.1242/dev.126.10.2181. [DOI] [PubMed] [Google Scholar]