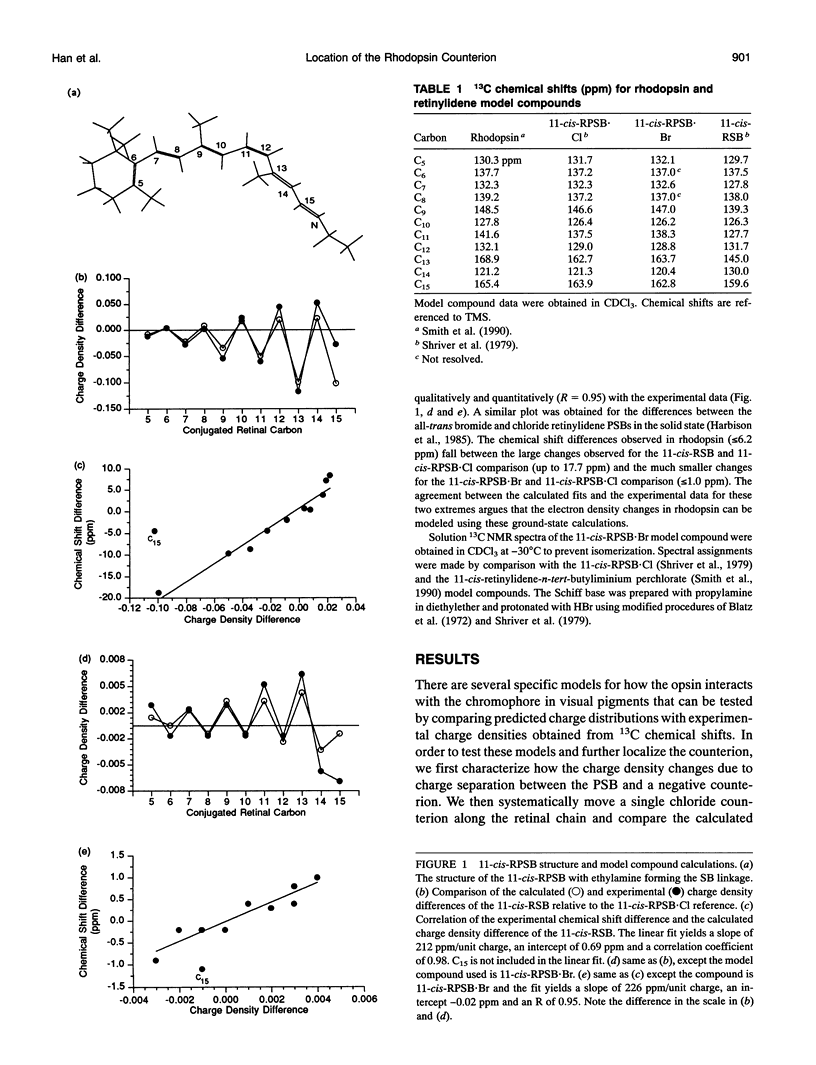
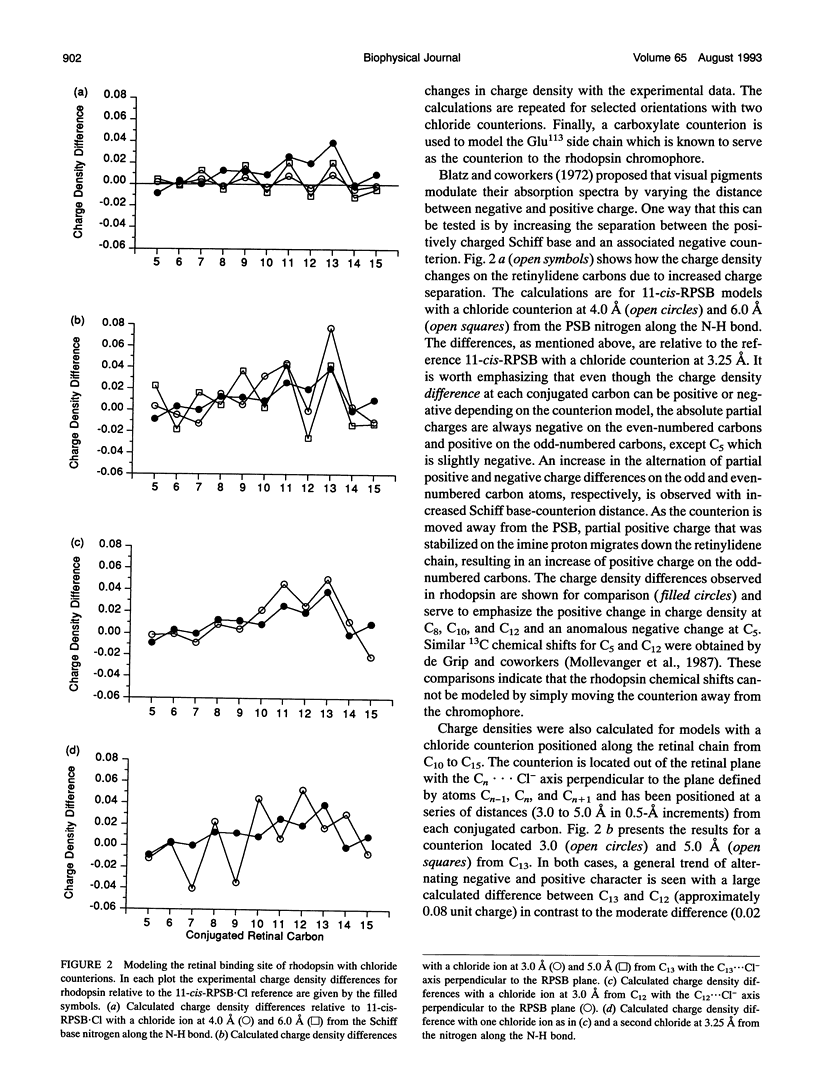
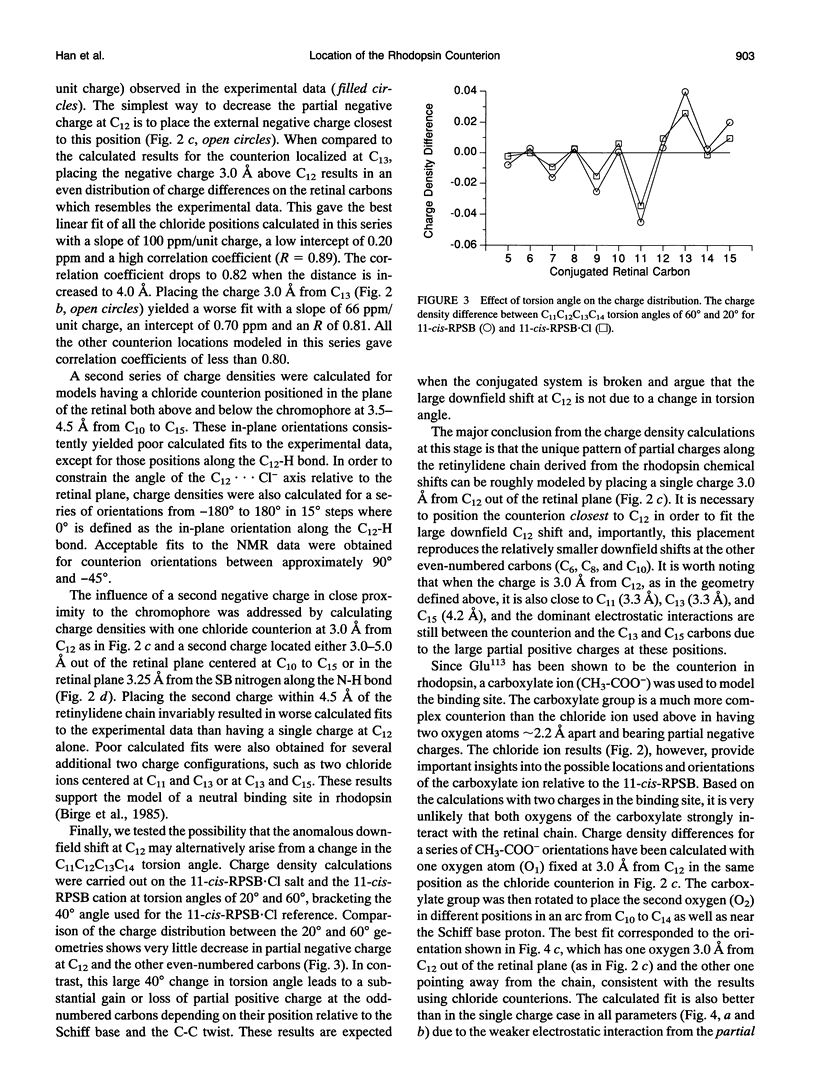
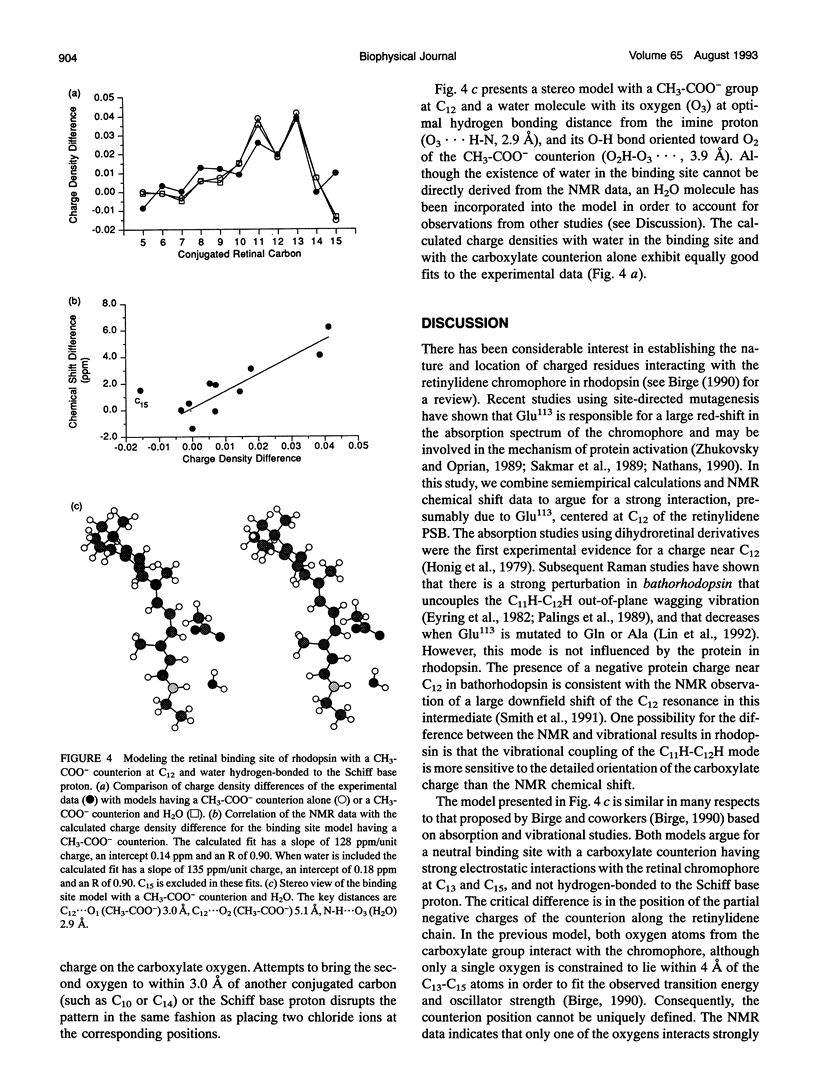
Abstract
Semiempirical molecular orbital calculations are combined with 13C NMR chemical shifts to localize the counterion in the retinal binding site of vertebrate rhodopsin. Charge densities along the polyene chain are calculated for an 11-cis-retinylidene protonated Schiff base (11-cis-RPSB) chromophore with 1) a chloride counterion at various distances from the Schiff base nitrogen, 2) one or two chloride counterions at different positions along the retinal chain from C10 to C15 and at the Schiff base nitrogen, and 3) a carboxylate counterion out of the retinal plane near C12. Increasing the distance of the negative counterion from the Schiff base results in an enhancement of alternating negative and positive partial charge on the even- and odd-numbered carbons, respectively, when compared to the 11-cis-RPSB chloride model compound. In contrast, the observed 13C NMR data of rhodopsin exhibit downfield chemical shifts from C8 to C13 relative to the 11-cis-RPSB.Cl corresponding to a net increase of partial positive or decrease of partial negative charge at these positions (Smith, S. O., I. Palings, M. E. Miley, J. Courtin, H. de Groot, J. Lugtenburg, R. A. Mathies, and R. G. Griffin. 1990. Biochemistry. 29:8158-8164). The anomalous changes in charge density reflected in the rhodopsin NMR chemical shifts can be qualitatively modeled by placing a single negative charge above C12. The calculated fit improves when a carboxylate counterion is used to model the retinal binding site. Inclusion of water in the model does not alter the fit to the NMR data, although it is consistent with observations based on other methods. These data constrain the location and the orientation of the Glu113 side chain, which is known to be the counterion in rhodopsin, and argue for a strong interaction centered at C12 of the retinylidene chain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baasov T., Friedman N., Sheves M. Factors affecting the C = N stretching in protonated retinal Schiff base: a model study for bacteriorhodopsin and visual pigments. Biochemistry. 1987 Jun 2;26(11):3210–3217. doi: 10.1021/bi00385a041. [DOI] [PubMed] [Google Scholar]
- Bagley K. A., Balogh-Nair V., Croteau A. A., Dollinger G., Ebrey T. G., Eisenstein L., Hong M. K., Nakanishi K., Vittitow J. Fourier-transform infrared difference spectroscopy of rhodopsin and its photoproducts at low temperature. Biochemistry. 1985 Oct 22;24(22):6055–6071. doi: 10.1021/bi00343a006. [DOI] [PubMed] [Google Scholar]
- Birge R. R., Einterz C. M., Knapp H. M., Murray L. P. The nature of the primary photochemical events in rhodopsin and isorhodopsin. Biophys J. 1988 Mar;53(3):367–385. doi: 10.1016/S0006-3495(88)83114-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R., Murray L. P., Pierce B. M., Akita H., Balogh-Nair V., Findsen L. A., Nakanishi K. Two-photon spectroscopy of locked-11-cis-rhodopsin: evidence for a protonated Schiff base in a neutral protein binding site. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4117–4121. doi: 10.1073/pnas.82.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim Biophys Acta. 1990 Apr 26;1016(3):293–327. doi: 10.1016/0005-2728(90)90163-x. [DOI] [PubMed] [Google Scholar]
- Blatz P. E., Mohler J. H., Navangul H. V. Anion-induced wavelength regulation of absorption maxima of Schiff bases of retinal. Biochemistry. 1972 Feb 29;11(5):848–855. doi: 10.1021/bi00755a026. [DOI] [PubMed] [Google Scholar]
- Eyring G., Curry B., Broek A., Lugtenburg J., Mathies R. Assignment and interpretation of hydrogen out-of-plane vibrations in the resonance Raman spectra of rhodopsin and bathorhodopsin. Biochemistry. 1982 Jan 19;21(2):384–393. doi: 10.1021/bi00531a028. [DOI] [PubMed] [Google Scholar]
- Gilson H. S., Honig B. H., Croteau A., Zarrilli G., Nakanishi K. Analysis of the factors that influence the C=N stretching frequency of polyene Schiff bases. Implications for bacteriorhodopsin and rhodopsin. Biophys J. 1988 Feb;53(2):261–269. doi: 10.1016/S0006-3495(88)83087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Greenberg A. D., Dinur U., Ebrey T. G. Visual-pigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry. 1976 Oct 19;15(21):4593–4599. doi: 10.1021/bi00666a008. [DOI] [PubMed] [Google Scholar]
- Honig B., Karplus M. Implications of torsional potential of retinal isomers for visual excitation. Nature. 1971 Feb 19;229(5286):558–560. doi: 10.1038/229558a0. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Tokitô Y., Chûjô R., Miyoshi Y. A study of pi-electron delocalization in model compounds of visual pigment by UV and carbon-13 NMR spectra. J Am Chem Soc. 1977 Aug 17;99(17):5592–5596. doi: 10.1021/ja00459a010. [DOI] [PubMed] [Google Scholar]
- KROPF A., HUBBARD R. The mechanism of bleaching rhodopsin. Ann N Y Acad Sci. 1959 Nov 12;74(2):266–280. doi: 10.1111/j.1749-6632.1958.tb39550.x. [DOI] [PubMed] [Google Scholar]
- Lin S. W., Sakmar T. P., Franke R. R., Khorana H. G., Mathies R. A. Resonance Raman microprobe spectroscopy of rhodopsin mutants: effect of substitutions in the third transmembrane helix. Biochemistry. 1992 Jun 9;31(22):5105–5111. doi: 10.1021/bi00137a003. [DOI] [PubMed] [Google Scholar]
- Mathies R., Oseroff A. R., Stryer L. Rapid-flow resonance Raman spectroscopy of photolabile molecules: rhodopsin and isorhodopsin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):1–5. doi: 10.1073/pnas.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollevanger L. C., Kentgens A. P., Pardoen J. A., Courtin J. M., Veeman W. S., Lugtenburg J., de Grip W. J. High-resolution solid-state 13C-NMR study of carbons C-5 and C-12 of the chromophore of bovine rhodopsin. Evidence for a 6-S-cis conformation with negative-charge perturbation near C-12. Eur J Biochem. 1987 Feb 16;163(1):9–14. doi: 10.1111/j.1432-1033.1987.tb10729.x. [DOI] [PubMed] [Google Scholar]
- Narva D., Callender R. H. On the state of chromophore protonation in rhodopsin: implication for primary photochemistry in visual pigments. Photochem Photobiol. 1980 Aug;32(2):273–276. doi: 10.1111/j.1751-1097.1980.tb04021.x. [DOI] [PubMed] [Google Scholar]
- Nathans J. Determinants of visual pigment absorbance: identification of the retinylidene Schiff's base counterion in bovine rhodopsin. Biochemistry. 1990 Oct 16;29(41):9746–9752. doi: 10.1021/bi00493a034. [DOI] [PubMed] [Google Scholar]
- Nathans J. Rhodopsin: structure, function, and genetics. Biochemistry. 1992 Jun 2;31(21):4923–4931. doi: 10.1021/bi00136a001. [DOI] [PubMed] [Google Scholar]
- Palings I., van den Berg E. M., Lugtenburg J., Mathies R. A. Complete assignment of the hydrogen out-of-plane wagging vibrations of bathorhodopsin: chromophore structure and energy storage in the primary photoproduct of vision. Biochemistry. 1989 Feb 21;28(4):1498–1507. doi: 10.1021/bi00430a012. [DOI] [PubMed] [Google Scholar]
- Rafferty C. N., Shichi H. The involvement of water at the retinal binding site in rhodopsin and early light-induced intramolecular proton transfer. Photochem Photobiol. 1981 Feb;33(2):229–234. doi: 10.1111/j.1751-1097.1981.tb05329.x. [DOI] [PubMed] [Google Scholar]
- Sakmar T. P., Franke R. R., Khorana H. G. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver J. W., Mateescu G. D., Abrahamson E. W. A proton and carbon-13 nuclear magnetic resonance spectroscopy study of the conformation of a protonated 11-cis-retinal Schiff base. Biochemistry. 1979 Oct 30;18(22):4785–4792. doi: 10.1021/bi00589a004. [DOI] [PubMed] [Google Scholar]
- Shriver J., Abrahamson E. W., Mateescu G. D. The structure of visual pigments. I. Carbon-13 nuclear magnetic resonance spectroscopy of N-all-trans-retinylidenepropylimine and its protonated species. J Am Chem Soc. 1976 Apr 28;98(9):2407–2409. doi: 10.1021/ja00425a006. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Courtin J., de Groot H., Gebhard R., Lugtenburg J. 13C magic-angle spinning NMR studies of bathorhodopsin, the primary photoproduct of rhodopsin. Biochemistry. 1991 Jul 30;30(30):7409–7415. doi: 10.1021/bi00244a007. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Palings I., Miley M. E., Courtin J., de Groot H., Lugtenburg J., Mathies R. A., Griffin R. G. Solid-state NMR studies of the mechanism of the opsin shift in the visual pigment rhodopsin. Biochemistry. 1990 Sep 4;29(35):8158–8164. doi: 10.1021/bi00487a025. [DOI] [PubMed] [Google Scholar]
- Tavan P., Schulten K., Oesterhelt D. The effect of protonation and electrical interactions on the stereochemistry of retinal schiff bases. Biophys J. 1985 Mar;47(3):415–430. doi: 10.1016/S0006-3495(85)83933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Karplus M. Calculation of pi-pi excited state conformations and vibronic structure of retinal and related molecules. J Am Chem Soc. 1974 Sep 4;96(18):5677–5689. doi: 10.1021/ja00825a001. [DOI] [PubMed] [Google Scholar]
- Zhukovsky E. A., Oprian D. D. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989 Nov 17;246(4932):928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]


