Abstract
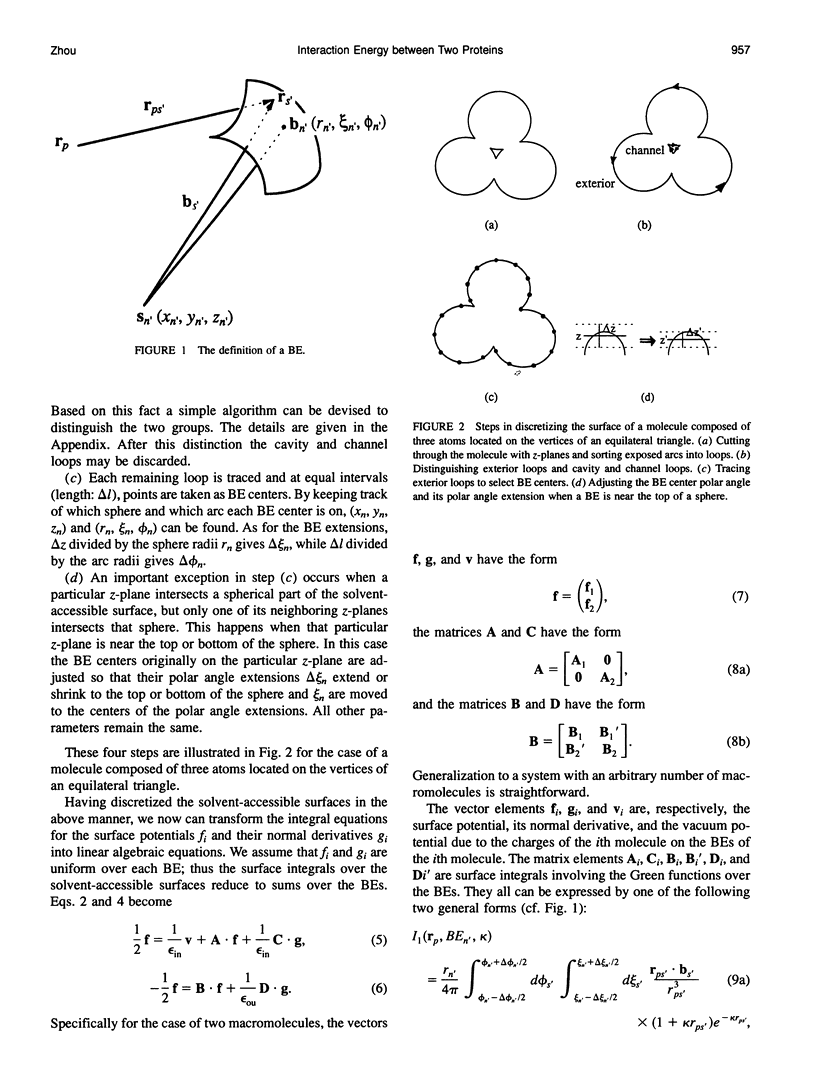
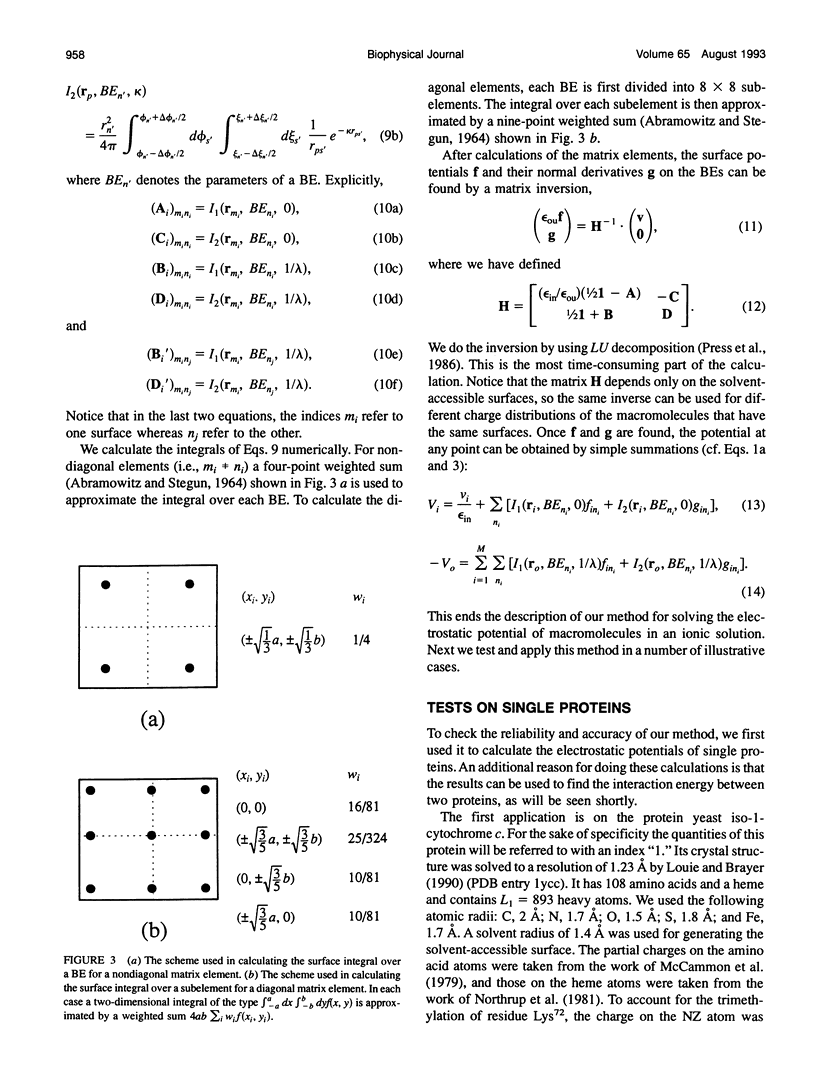
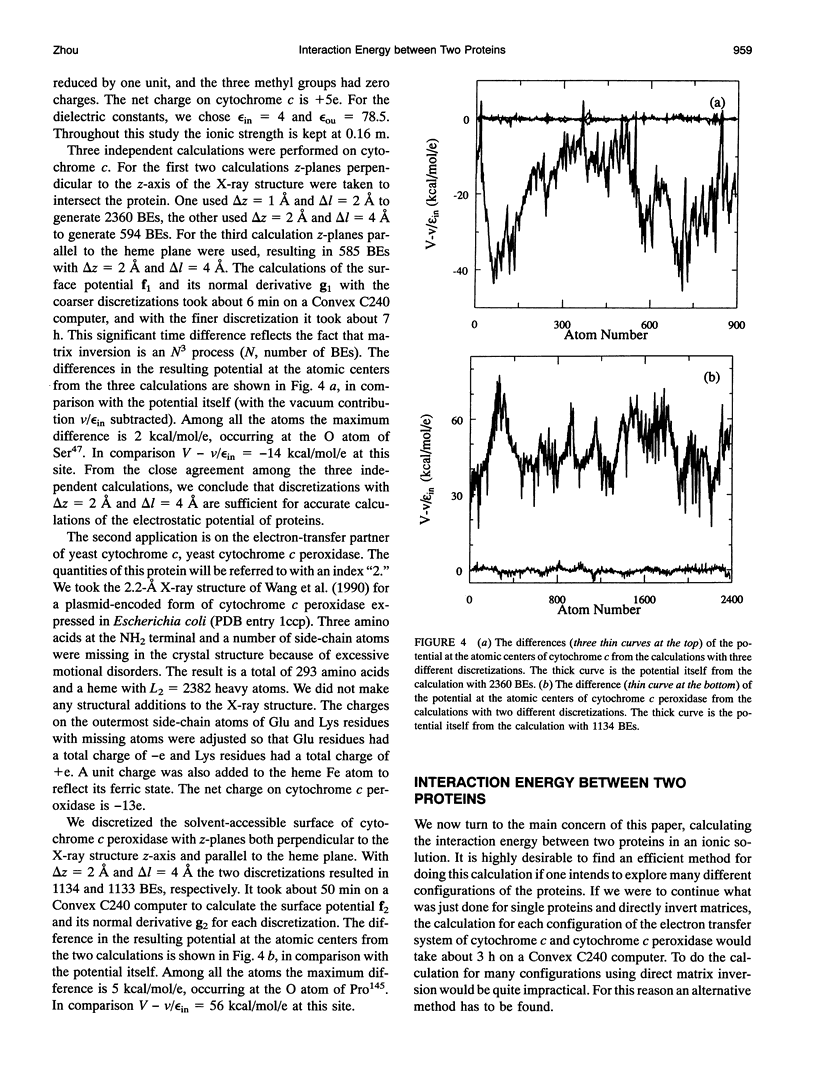
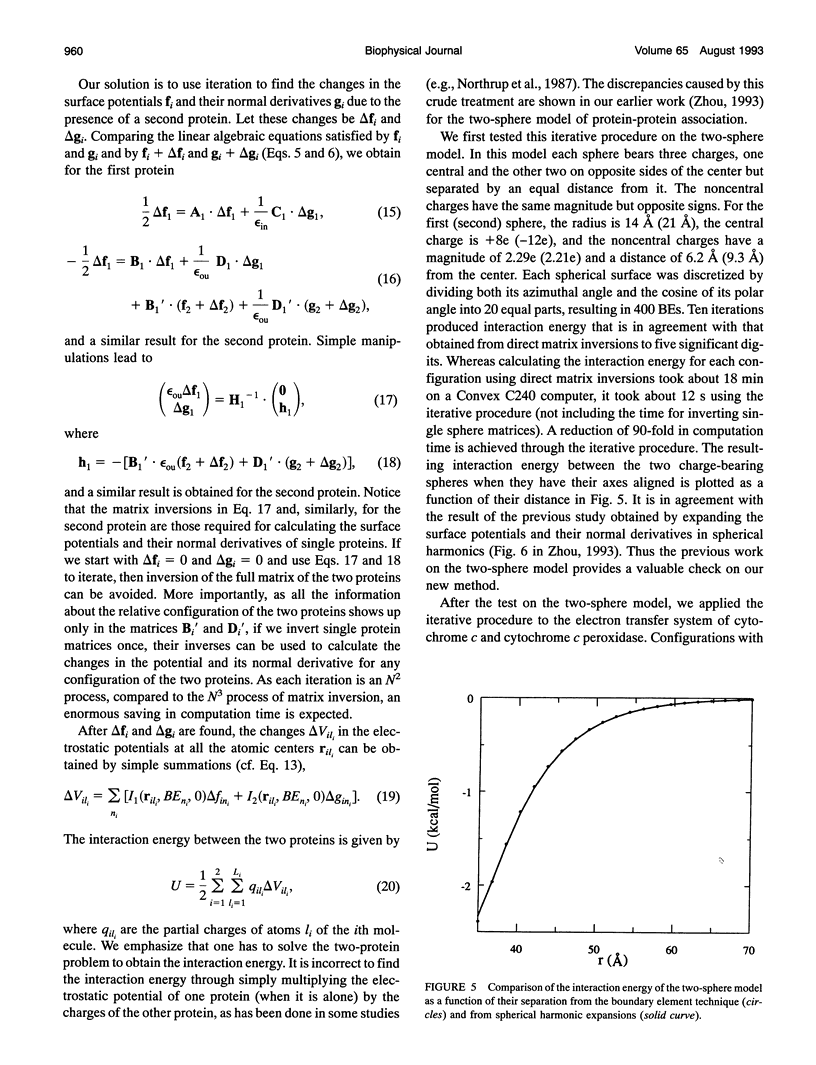
The boundary element technique is implemented to solve for the electrostatic potential of macromolecules in an ionic solution. This technique entails solving surface integral equations that are equivalent to the Poisson and the Poisson-Boltzmann equations governing the electrostatic potential inside the macromolecules and and in the solvent. A simple but robust method is described for discretizing the macromolecular surfaces in order to approximate the integral equations by linear algebraic equations. Particular attention is paid to the interaction energy between two macromolecules, and an iterative procedure is devised to make the calculation more efficient. This iterative procedure is illustrated in the electron transfer system of cytochrome c and cytochrome c peroxidase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Klapper I., Hagstrom R., Fine R., Sharp K., Honig B. Focusing of electric fields in the active site of Cu-Zn superoxide dismutase: effects of ionic strength and amino-acid modification. Proteins. 1986 Sep;1(1):47–59. doi: 10.1002/prot.340010109. [DOI] [PubMed] [Google Scholar]
- Louie G. V., Brayer G. D. High-resolution refinement of yeast iso-1-cytochrome c and comparisons with other eukaryotic cytochromes c. J Mol Biol. 1990 Jul 20;214(2):527–555. doi: 10.1016/0022-2836(90)90197-T. [DOI] [PubMed] [Google Scholar]
- McCammon J. A., Wolynes P. G., Karplus M. Picosecond dynamics of tyrosine side chains in proteins. Biochemistry. 1979 Mar 20;18(6):927–942. doi: 10.1021/bi00573a001. [DOI] [PubMed] [Google Scholar]
- Northrup S. H., Pear M. R., Morgan J. D., McCammon J. A., Karplus M. Molecular dynamics of ferrocytochrome c. Magnitude and anisotropy of atomic displacements. J Mol Biol. 1981 Dec 25;153(4):1087–1109. doi: 10.1016/0022-2836(81)90469-1. [DOI] [PubMed] [Google Scholar]
- Pelletier H., Kraut J. Crystal structure of a complex between electron transfer partners, cytochrome c peroxidase and cytochrome c. Science. 1992 Dec 11;258(5089):1748–1755. doi: 10.1126/science.1334573. [DOI] [PubMed] [Google Scholar]
- Warwicker J., Watson H. C. Calculation of the electric potential in the active site cleft due to alpha-helix dipoles. J Mol Biol. 1982 Jun 5;157(4):671–679. doi: 10.1016/0022-2836(82)90505-8. [DOI] [PubMed] [Google Scholar]
- Zauhar R. J., Morgan R. S. A new method for computing the macromolecular electric potential. J Mol Biol. 1985 Dec 20;186(4):815–820. doi: 10.1016/0022-2836(85)90399-7. [DOI] [PubMed] [Google Scholar]
- Zhou H. X. Brownian dynamics study of the influences of electrostatic interaction and diffusion on protein-protein association kinetics. Biophys J. 1993 Jun;64(6):1711–1726. doi: 10.1016/S0006-3495(93)81543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


