Abstract
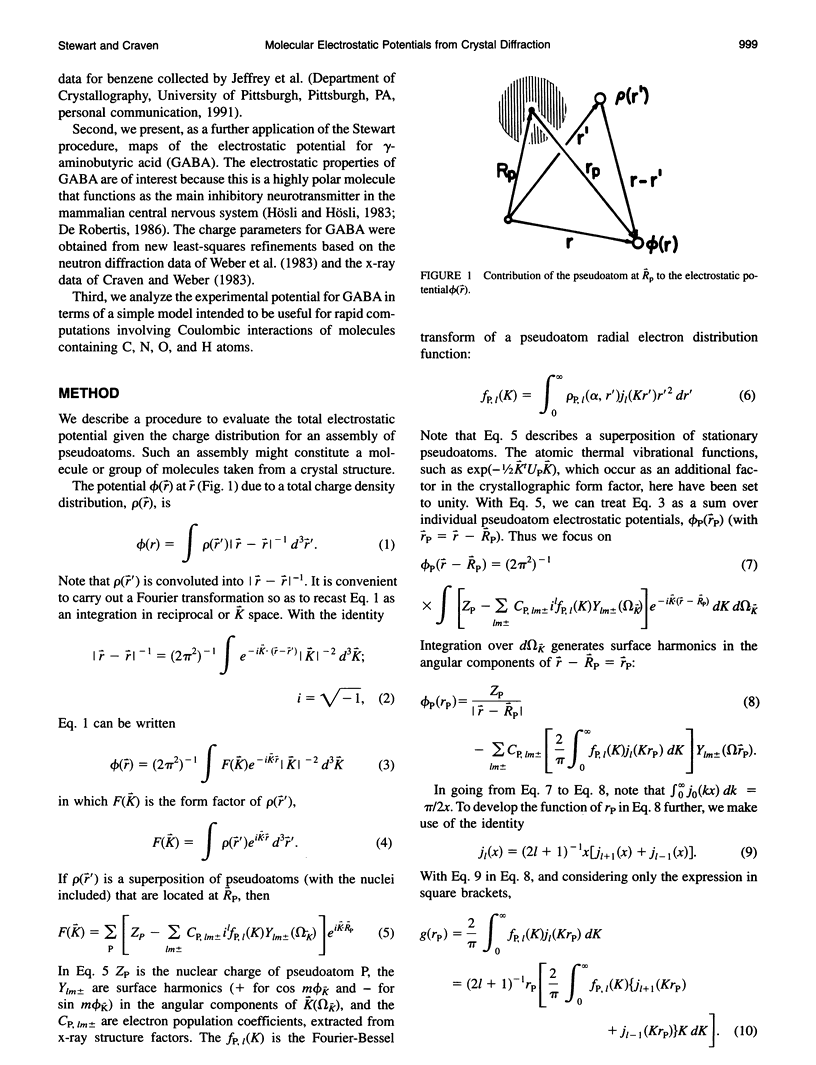
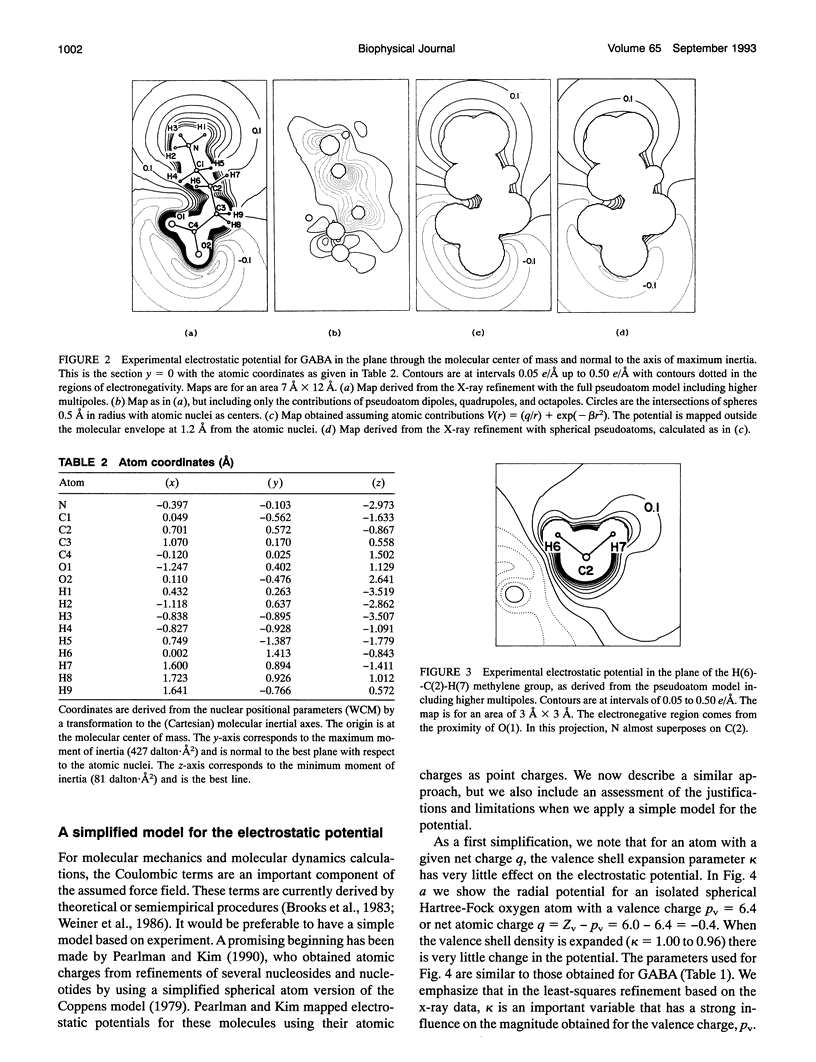
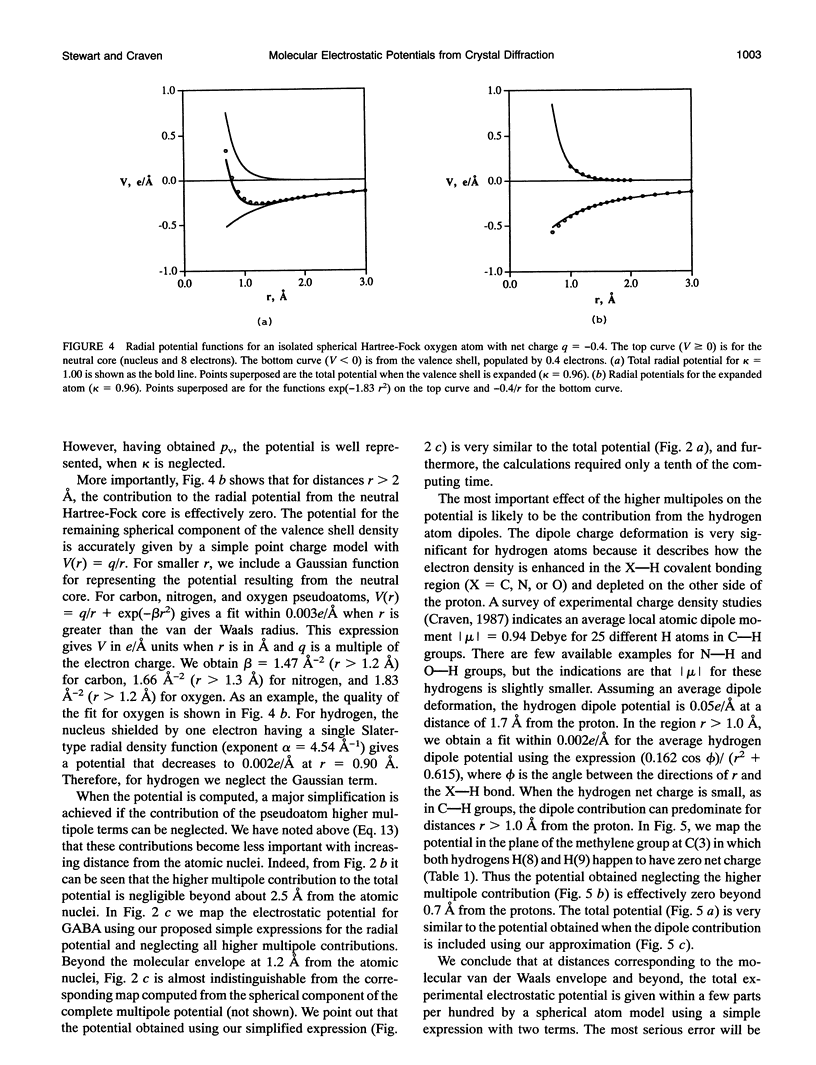
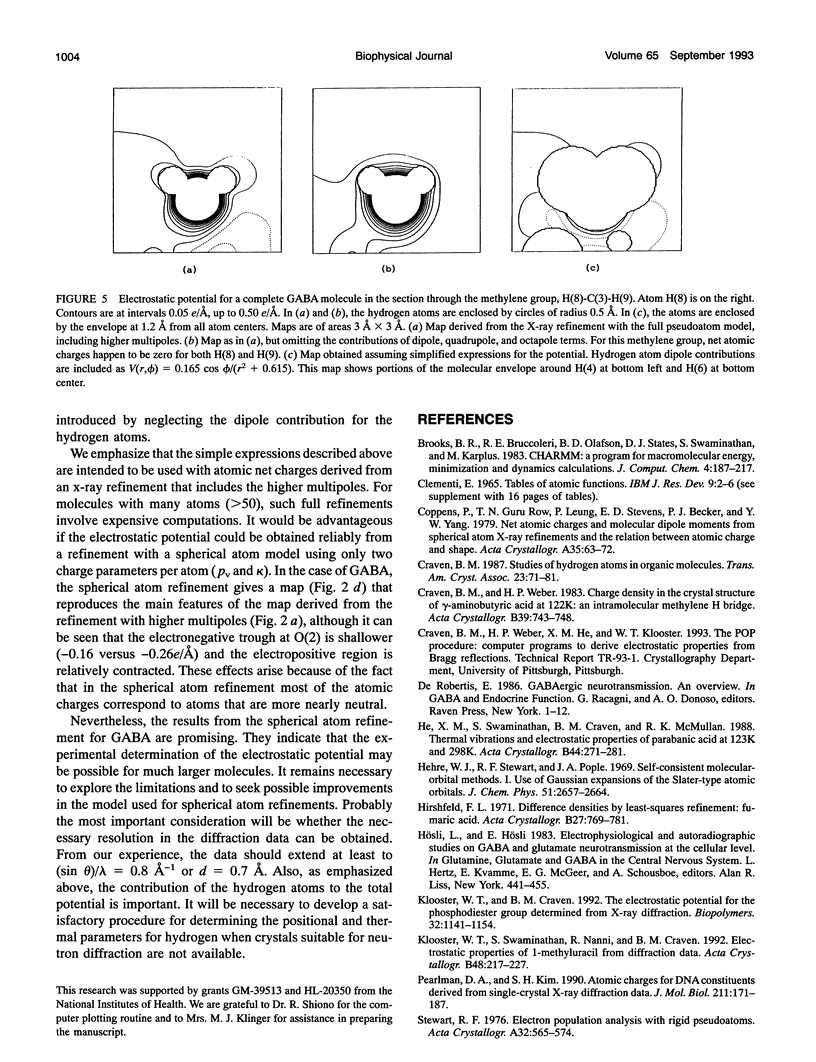
Given the electronic charge parameters obtained from a diffraction study of the charge density distribution in a crystal, a mathematical procedure is presented for deriving the electrostatic potential. The procedure allows the mapping of electrostatic potential for a molecule or group of molecules removed from the crystal structure but with each molecule retaining the effects of polarization owing to the original crystal environment. The method is applied for the neurotransmitter gamma-aminobutyric acid. The potential for a gamma-aminobutyric acid molecule is analyzed in terms of a simple model that is suitable for rapid computations concerned with Coulombic molecular interactions. Outside the molecular envelope at 1.2 A from the atomic nuclei, the total potential is well represented by a sum of spherical atom contributions with V(r) = (q/r) + exp(-beta r2). The most important aspherical component in the potential is the dipole contribution from the hydrogen atoms. This can be represented as V(r, phi) = (0.162 cos phi)/(r2 + 0.615). Here, V is in e/A, r is the distance from each nucleus in A, q is the experimentally determined net atomic charge in electron units, and phi is the angle between r and the bond X-H. We obtain beta = 1.47, 1.66, 1.83 A-2 for C, N, and O respectively. For H, no term in beta is needed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- He X. M., Swaminathan S., Craven B. M., McMullan R. K. Thermal vibrations and electrostatic properties of parabanic acid at 123 and 298 K. Acta Crystallogr B. 1988 Jun 1;44(Pt 3):271–281. doi: 10.1107/s0108768187011108. [DOI] [PubMed] [Google Scholar]
- Klooster W. T., Craven B. M. The electrostatic potential for the phosphodiester group determined from X-ray diffraction. Biopolymers. 1992 Sep;32(9):1141–1154. doi: 10.1002/bip.360320903. [DOI] [PubMed] [Google Scholar]
- Klooster W. T., Swaminathan S., Nanni R., Craven B. M. Electrostatic properties of 1-methyluracil from diffraction data. Acta Crystallogr B. 1992 Apr 1;48(Pt 2):217–227. doi: 10.1107/s0108768191013319. [DOI] [PubMed] [Google Scholar]
- Pearlman D. A., Kim S. H. Atomic charges for DNA constituents derived from single-crystal X-ray diffraction data. J Mol Biol. 1990 Jan 5;211(1):171–187. doi: 10.1016/0022-2836(90)90019-I. [DOI] [PubMed] [Google Scholar]
- Su Z., Coppens P. On the mapping of electrostatic properties from the multipole description of the charge density. Acta Crystallogr A. 1992 Mar 1;48(Pt 2):188–197. doi: 10.1107/s0108767391009820. [DOI] [PubMed] [Google Scholar]


