Abstract
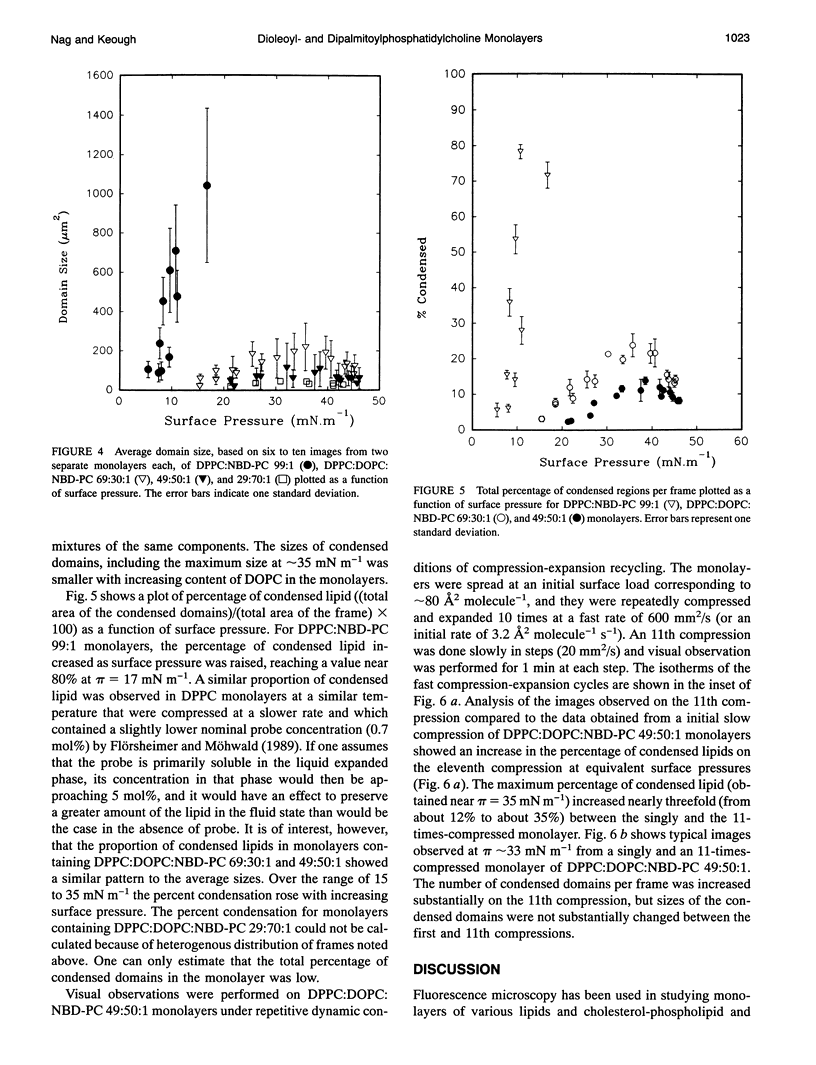
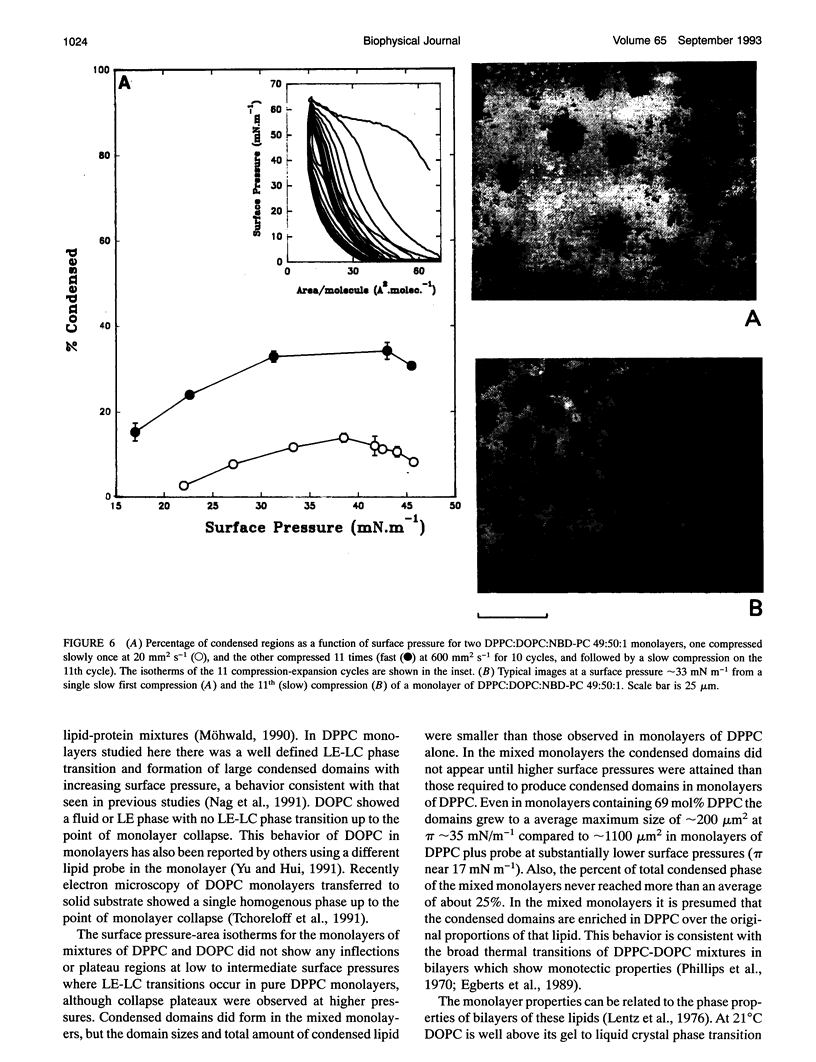
Monolayers of dipalmitoylphosphatidylcholine (DPPC), dioleoylphosphatidylcholine (DOPC), and some mixtures of these lipids were investigated using an epifluorescence microscopic surface balance. Monolayers were visualized at 23 +/- 1 degree C through the fluorescence of 1 mol% of two different fluorescent probes, 1-palmitoyl-2-(12-[(7-nitro-2-1,3-benzoxadizole-4- yl)amino]dodecanoyl)phosphatidylcholine (NBD-PC), which partitions into the liquid expanded (LE) or disordered lipid phase and 3,3'-dioctadecyloxacarbocyanine perchlorate (DiO-C18), which preferentially associates with the liquid condensed (LC) phase or lipid with ordered chains. LC domains were observed in pure DPPC monolayers at relatively low surface pressures (pi), and these domains grew with increasing surface pressure. Only liquid expanded phase was observed in pure DOPC monolayers up to the point of monolayer collapse. In monolayers containing 29:70:1, 49:50:1, and 69:30:1 (mol/mol/mol) of DPPC:DOPC:probe the domains of LC phase were smaller than those seen in DPPC monolayers at equivalent surface pressures. Quantitative analysis of the visual fields shown by the mixed monolayers showed a distribution of sizes of condensed domains at any given pi. At pi = 30 mN m-1, liquid-expanded, or fluid, regions occupied more than 70% of the total monolayer area in all three mixtures studied, whereas DPPC monolayers were more than 75% condensed or solid at that pressure. For monolayers of DPPC:DOPC:NBD-PC 49:50:1 and 69:30:1 the average domain size and the percentage of the total area covered with LC, or rigid, areas increased to a maximum at pi around 35 mN m-1 followed by a decrease at higher pi. Repetitive compression and expansion of the monolayers containing DPPC:DOPC:NBD-PC 49:50:1 at an initial rate of 3.2 A2 molecule-1 s-1 produced monolayers with visual properties consistent with there being a preferential exclusion of the unsaturated lipid from the monolayer.
Full text
PDF
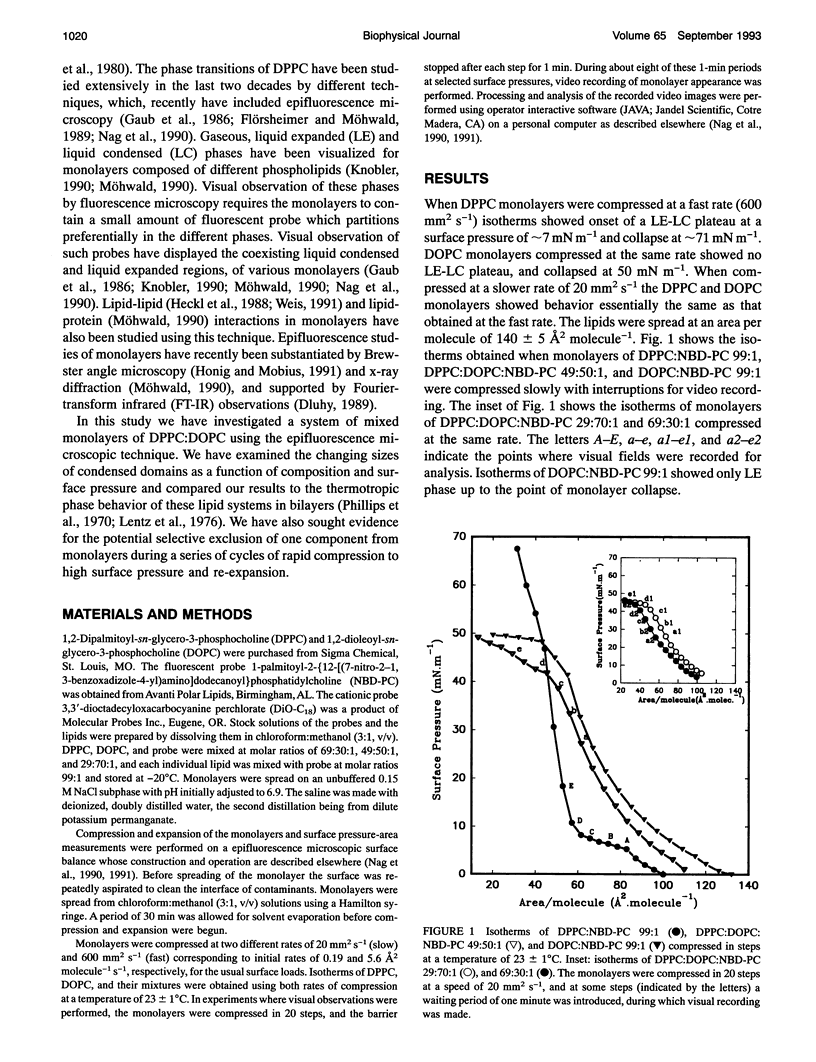
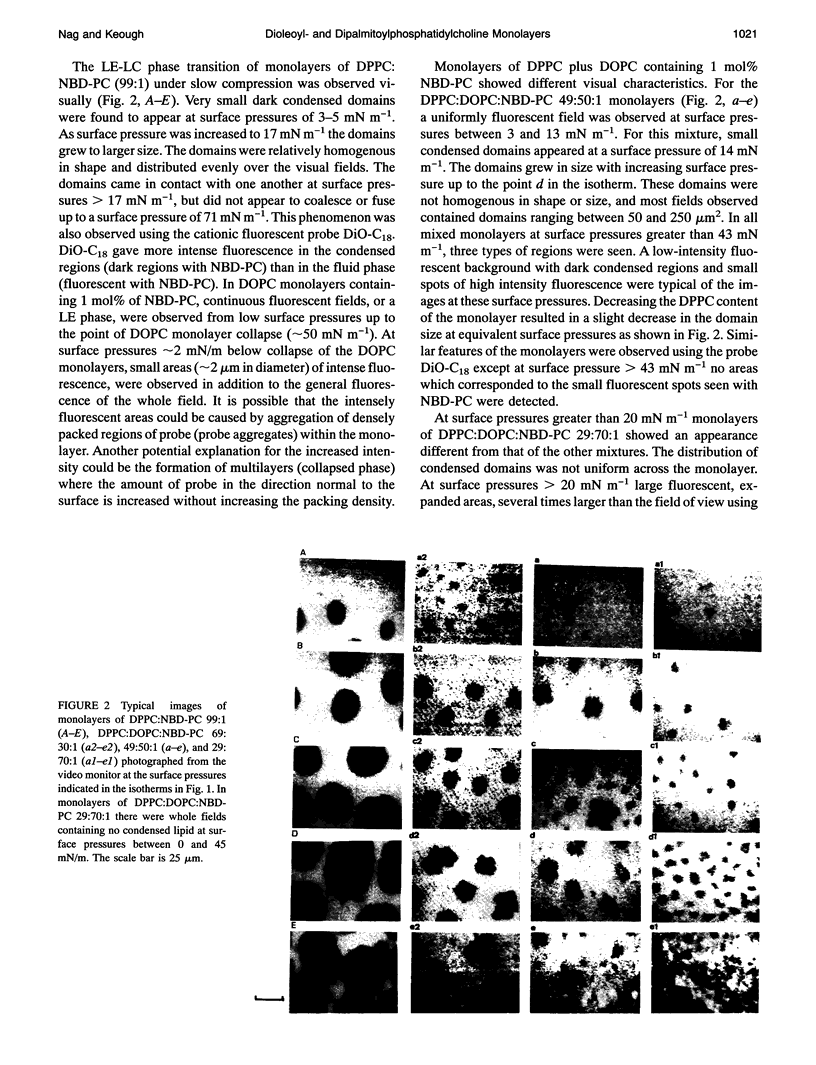
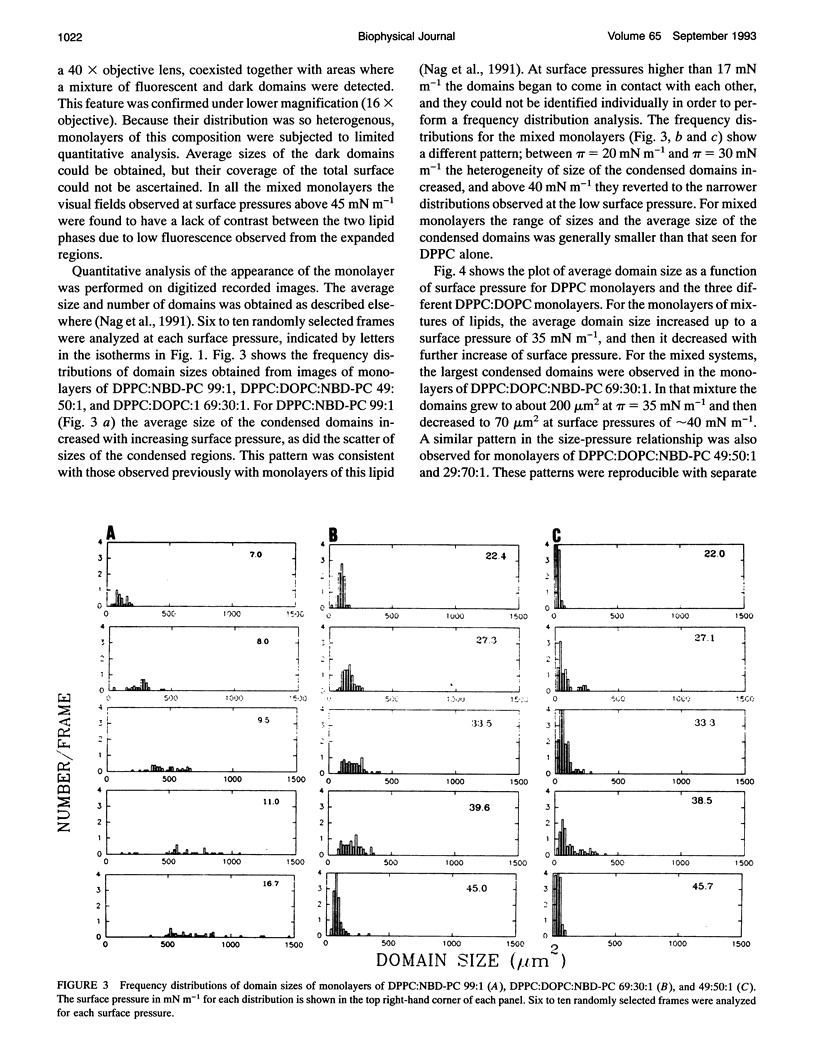


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham A. D., Morley C. J., Phillips M. C. The physical properties of an effective lung surfactant. Biochim Biophys Acta. 1979 Jun 21;573(3):552–556. doi: 10.1016/0005-2760(79)90229-7. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Morgan C. G., Radda G. K. Measurement and interpretation of fluorescence polarisations in phospholipid dispersions. Biochim Biophys Acta. 1976 Mar 5;426(2):157–172. doi: 10.1016/0005-2736(76)90329-1. [DOI] [PubMed] [Google Scholar]
- Blume A. A comparative study of the phase transitions of phospholipid bilayers and monolayers. Biochim Biophys Acta. 1979 Oct 19;557(1):32–44. doi: 10.1016/0005-2736(79)90087-7. [DOI] [PubMed] [Google Scholar]
- Curstedt T., Jörnvall H., Robertson B., Bergman T., Berggren P. Two hydrophobic low-molecular-mass protein fractions of pulmonary surfactant. Characterization and biophysical activity. Eur J Biochem. 1987 Oct 15;168(2):255–262. doi: 10.1111/j.1432-1033.1987.tb13414.x. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Coolbear K. P., Keough K. M. Differential scanning calorimetric studies of the thermotropic phase behavior of membranes composed of dipalmitoyllecithin and mixed-acid unsaturated lecithins. Can J Biochem. 1980 Oct;58(10):851–858. doi: 10.1139/o80-118. [DOI] [PubMed] [Google Scholar]
- Dluhy R. A., Reilly K. E., Hunt R. D., Mitchell M. L., Mautone A. J., Mendelsohn R. Infrared spectroscopic investigations of pulmonary surfactant. Surface film transitions at the air-water interface and bulk phase thermotropism. Biophys J. 1989 Dec;56(6):1173–1181. doi: 10.1016/S0006-3495(89)82764-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egberts J., Sloot H., Mazure A. Minimal surface tension, squeeze-out and transition temperatures of binary mixtures of dipalmitoylphosphatidylcholine and unsaturated phospholipids. Biochim Biophys Acta. 1989 Mar 14;1002(1):109–113. doi: 10.1016/0005-2760(89)90072-6. [DOI] [PubMed] [Google Scholar]
- Flörsheimer M., Möhwald H. Development of equilibrium domain shapes in phospholipid monolayers. Chem Phys Lipids. 1989 Mar;49(4):231–241. doi: 10.1016/0009-3084(89)90071-6. [DOI] [PubMed] [Google Scholar]
- Haverstick D. M., Glaser M. Influence of proteins on the reorganization of phospholipid bilayers into large domains. Biophys J. 1989 Apr;55(4):677–682. doi: 10.1016/S0006-3495(89)82866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawco M. W., Coolbear K. P., Davis P. J., Keough K. M. Exclusion of fluid lipid during compression of monolayers of mixtures of dipalmitoylphosphatidylcholine with some other phosphatidylcholines. Biochim Biophys Acta. 1981 Aug 6;646(1):185–187. doi: 10.1016/0005-2736(81)90286-8. [DOI] [PubMed] [Google Scholar]
- Hawco M. W., Davis P. J., Keough K. M. Lipid fluidity in lung surfactant: monolayers of saturated and unsaturated lecithins. J Appl Physiol Respir Environ Exerc Physiol. 1981 Aug;51(2):509–515. doi: 10.1152/jappl.1981.51.2.509. [DOI] [PubMed] [Google Scholar]
- Hildebran J. N., Goerke J., Clements J. A. Pulmonary surface film stability and composition. J Appl Physiol Respir Environ Exerc Physiol. 1979 Sep;47(3):604–611. doi: 10.1152/jappl.1979.47.3.604. [DOI] [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol. 1972 Sep;223(3):715–726. doi: 10.1152/ajplegacy.1972.223.3.715. [DOI] [PubMed] [Google Scholar]
- Knobler C. M. Seeing phenomena in flatland: studies of monolayers by fluorescence microscopy. Science. 1990 Aug 24;249(4971):870–874. doi: 10.1126/science.249.4971.870. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 2 Two-component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4529–4537. doi: 10.1021/bi00665a030. [DOI] [PubMed] [Google Scholar]
- Möhwald H. Phospholipid and phospholipid-protein monolayers at the air/water interface. Annu Rev Phys Chem. 1990;41:441–476. doi: 10.1146/annurev.pc.41.100190.002301. [DOI] [PubMed] [Google Scholar]
- Nag K., Boland C., Rich N., Keough K. M. Epifluorescence microscopic observation of monolayers of dipalmitoylphosphatidylcholine: dependence of domain size on compression rates. Biochim Biophys Acta. 1991 Sep 30;1068(2):157–160. doi: 10.1016/0005-2736(91)90204-l. [DOI] [PubMed] [Google Scholar]
- Notter R. H., Tabak S. A., Mavis R. D. Surface properties of binary mixtures of some pulmonary surfactant components. J Lipid Res. 1980 Jan;21(1):10–22. [PubMed] [Google Scholar]
- Phillips M. C., Ladbrooke B. D., Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970 Jan 6;196(1):35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- Schürch S., Bachofen H., Goerke J., Possmayer F. A captive bubble method reproduces the in situ behavior of lung surfactant monolayers. J Appl Physiol (1985) 1989 Dec;67(6):2389–2396. doi: 10.1152/jappl.1989.67.6.2389. [DOI] [PubMed] [Google Scholar]
- Schürch S., Goerke J., Clements J. A. Direct determination of surface tension in the lung. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4698–4702. doi: 10.1073/pnas.73.12.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoreloff P., Gulik A., Denizot B., Proust J. E., Puisieux F. A structural study of interfacial phospholipid and lung surfactant layers by transmission electron microscopy after Blodgett sampling: influence of surface pressure and temperature. Chem Phys Lipids. 1991 Sep;59(2):151–165. doi: 10.1016/0009-3084(91)90004-u. [DOI] [PubMed] [Google Scholar]
- Weis R. M. Fluorescence microscopy of phospholipid monolayer phase transitions. Chem Phys Lipids. 1991 Mar;57(2-3):227–239. doi: 10.1016/0009-3084(91)90078-p. [DOI] [PubMed] [Google Scholar]
- Wildeboer-Venema F. A model for the study of the physical behaviour of the lung-surfactant film in vitro. Respir Physiol. 1978 Feb;32(2):225–237. doi: 10.1016/0034-5687(78)90112-3. [DOI] [PubMed] [Google Scholar]