Abstract
The Tongoleleka archaeological site on Lifuka Island, Kingdom of Tonga, is a rich accumulation of pottery, marine mollusks, and nonhuman bones that represents first human contact on a small island in Remote Oceania ≈2,850 years ago. The lower strata contain decorated Lapita-style pottery and bones of an extinct iguana (Brachylophus undescribed sp.) and numerous species of extinct birds. The upper strata instead feature Polynesian Plainware pottery and bones of extant species of vertebrates. A stratigraphic series of 20 accelerator-mass spectrometer radiocarbon dates on individual bones of the iguana, an extinct megapode (Megapodius alimentum), and the non-native chicken (Gallus gallus) suggests that anthropogenic loss of the first two species and introduction of the latter occurred on Lifuka within a time interval too short (a century or less) to be resolved by radiometric dating. The geologically instantaneous prehistoric collapse of Lifuka's vertebrate community contrasts with the much longer periods of faunal depletion on some other islands, thus showing that the elapse time between human arrival and major extinction events was highly variable on oceanic islands as well as on continents.
Modern vertebrates on oceanic islands are, on average, more vulnerable to human-caused extinction than those on continents (1). This trend applied in prehistoric times as well. On most Polynesian islands, for example, more species of birds were lost prehistorically than survived to be recorded alive by scientists (2–5). For lizards in Oceania, a prehistoric record is only now emerging, but also suggests considerable extinction, especially among large-bodied species, after human colonization (6–8). Here we use radiocarbon dates on individual bones (identified to the species level) to show that these prehistoric extinctions occurred very rapidly on some islands, suggesting a “blitzkrieg” type of extinction event as proposed for late Pleistocene large mammals in Australia and North America (9, 10).
Materials and Methods
The Archaeological Site.
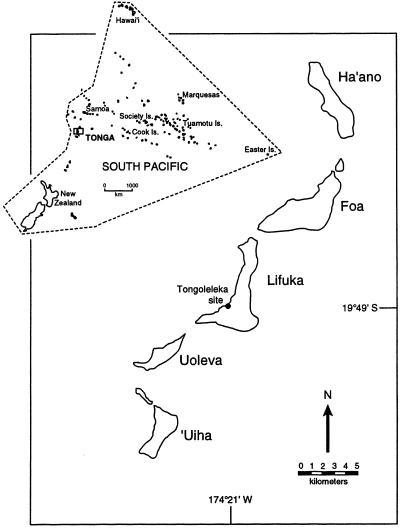
The Tongoleleka archaeological site (acronym Li7) lies in calcareous sands on the leeward coast of Lifuka Island, Ha'apai Group, Kingdom of Tonga (Fig. 1). Preliminary assessments of the stratigraphy, chronology, and cultural context of site Li7 were based on small excavations in 1984 and 1992 (11–15). More extensive excavations (11 m2) in 1995 and 1997, directed primarily by D.V.B., have yielded a much larger sample of materials from site Li7, including abundant prehistoric artifacts (pottery, shell beads and bracelets, and bone awls and needles) and associated nonhuman bones that represent indigenous species of marine fish (Osteichthyes, Chondrichthyes), sea turtles (Cheloniidae), small lizards (Gekkonidae, Scincidae), iguanas (Brachylophus undescribed sp.), birds (numerous species; see below), flying foxes (Pteropus spp.), and porpoises (Delphinidae), as well as four species introduced prehistorically, the chicken (Gallus gallus), Pacific rat (Rattus exulans), dog (Canis familiaris), and pig (Sus scrofa). Site Li7 also is a rich source of remains from marine invertebrates (gastropods, bivalves, coral, urchins, and crabs), with the first two especially abundant. The invertebrates have not yet been analyzed.
Figure 1.
Lifuka Island in the Ha'apai Group of Tonga, showing location of the Tongoleleka site.
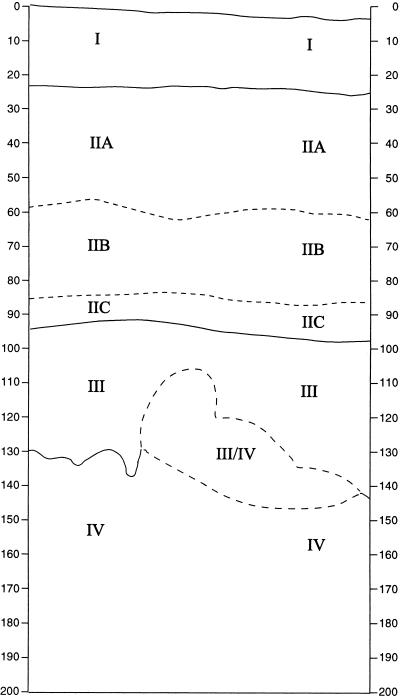
The sediments at site Li7 consist of four main strata, designated as Layers (Fig. 2). Layers I–III yielded abundant cultural materials, though these often were degraded in Layer I, a dark, compacted, clayey, silty topsoil. Layer II (divided into substrata IIA, IIB, and IIC because of minor textural and color changes) is a slightly sandy silt that is lighter in color and less compacted than Layer I. Layer III is even less compacted, lighter, and much sandier. Layer IV is culturally sterile calcareous beach sand. Layer III/IV, especially rich in bones, is a swirled deposit in which silty sand (Layer III) is mixed with beach sand (Layer IV).
Figure 2.
Stratigraphic profile of the east wall of excavation unit 10, Tongoleleka (site Li7), Lifuka, Tonga. Distribution of arbitrary levels (Arabic numbers) within the natural strata (Roman numerals) is as follows: Layer I, levels 1–4; Layer IIA, levels 5–10; Layer IIB, levels 11–15; Layer IIC, levels 16–19; Layer III, levels 20–28; Layer III/IV, levels 29–37, 40, and 42–45; Layer IV, levels 38, 39, and 41. Overlap in the numerical sequence of levels between Layer III/IV and Layer IV is because levels 37, 40, and 42–45 were a diagonal feature, not developed in the east wall, that intruded deep into Layer IV. Vertical scale is in centimeters below datum.
We believe that Layer III/IV represents the earliest human habitation of Lifuka. At Li7, as at every other Lapita archaeological site we have seen in Tonga (10 sites on six islands), the underlying calcareous beach sand is utterly devoid of artifacts, food remains, charcoal, or anything else that might represent human presence. These same attributes also apply to a cave site named ‘Anatu on ‘Eua in southern Tonga. At ‘Anatu, the silty sediments older than ≈2,800 calendar years (Cal) B.P. contained only remains of indigenous land snails and vertebrates, whereas the overlying sediments were markedly different in that they contained charcoal, ash, pit features, marine mollusks, and bones of humans, rats, dogs, pigs, and chickens (4).
The pottery at site Li7 includes the distinctive, dentate-stamped bowls, plates, and jars known as Lapita, varieties of which have been found on islands across 4,500 km of the western Pacific from the Bismarck Archipelago to Tonga and Samoa (16–18). New Caledonia, Vanuatu, Fiji, Tonga, and Samoa were uninhabited when Lapita people arrived 2,900 to 2,800 years ago, whereas the Bismarcks and nearby Solomon Islands, relatively close to New Guinea, already had been occupied by aceramic peoples for 20,000–30,000 years when the Lapita potters came ashore 3,500 to 3,200 years ago (19). The distribution of Lapita-style pottery (decorated potsherds) at Li7 is plotted in Fig. 3. The pottery style that replaces Lapita in Tongan sites is called Polynesian Plainware, although it is difficult or impossible to classify most nondecorated body sherds as being Lapita or Polynesian Plainware. Rim sherds that certainly or probably represent Polynesian Plainware are very rare in Layer III/IV, rare in Layer III, and common in Layers II and I. The transition from Lapita pottery to Polynesian Plainware is believed to represent an in situ change rather than the immigration of new peoples (13, 16, 17).
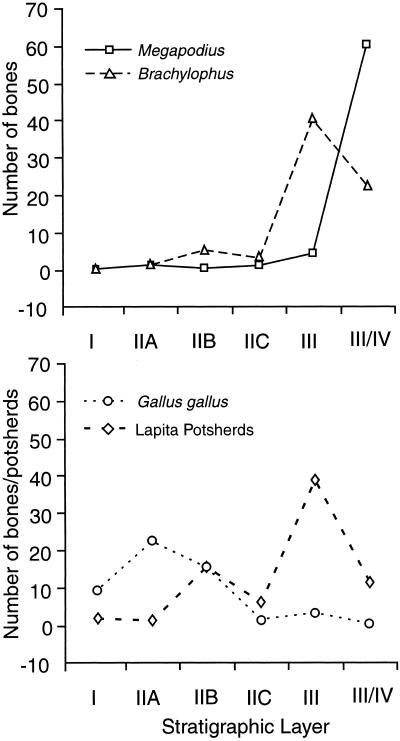
Figure 3.
Stratigraphic distribution of bones of megapodes (Megapodius alimentum), iguanas (Brachylophus undescribed sp.), and chicken (Gallus gallus), and decorated (Lapita) potsherds in Unit 10, Tongoleleka (site Li7), Lifuka, Tonga. Layer I is uppermost; Layer III/IV is the lowest cultural stratum.
Accelerator-Mass Spectrometer (AMS) Radiocarbon Dating.
This report is based on detailed excavation of a 1-m2 area (Unit 10) at site Li7 by D.W.S. in 1997, with the goal of obtaining bones from precise stratigraphic contexts for AMS radiocarbon (14C) dating. AMS 14C dates can be done on individual identified bones of small species because the method requires much less bone (as little as 15 mg, if well preserved chemically) than the 100–1,000 g needed for conventional β-decay 14C dating (20, 21). Thus, AMS 14C dating eliminates the uncertainty of whether remains of extinct vertebrates are truly associated with radiometrically dated nonvertebrate materials, such as charcoal. AMS 14C dating also has no problem with time-averaging, as long as each 14C date is based on a single bone rather than multiple bones (22).
Each of the AMS 14C dates reported herein is based on a single bone identified to the species level by D.W.S. and G.K.P. Six of these dates were on bones of the chicken G. gallus, native to southeast Asia but introduced prehistorically through much of Oceania. Eight dates were on bones of the indigenous, extinct megapode Megapodius alimentum, originally described from specimens collected at site Li7 (23) and now known from prehistoric sites on seven islands in Tonga and one in Fiji (24, 25). The other six dates were on bones of the indigenous, extinct iguana Brachylophus undescribed sp., known from archaeological sites on six Tongan islands. The extinct iguana was much larger than either of the two surviving species of Brachylophus (ref. 6 and G.K.P. unpublished data).
Among the three species used for the AMS 14C determinations, we are confident that each dated bone of G. gallus represents a different individual chicken because of stratigraphy and sexual dimorphism in size, with the two bones from Layer IIA representing a male (coracoid) and female (humerus). For the monomorphic M. alimentum, the eight dated bones represent five to eight individual megapodes. For Brachylophus undescribed sp., the six dated bones represent four to six individual iguanas.
Results
We determined AMS 14C dates on 18 individual bones from Unit 10 and 2 bones from the adjacent Unit 11 at site Li7 (Table 1). At 95% confidence, the calibrated 14C age of the stratigraphically highest dated sample (Level 5; Beta-134573, 2,935–2,755 Cal B.P.) is nearly identical to that of the deepest dated sample (Level 45; Beta-134585, 2,960–2,765 Cal B.P.). The youngest intercept is between 2,785 and 2,710 Cal B.P. in 18 of the 20 AMS 14C dates (Table 1), including the five uppermost dated samples (from Levels 5, 8, 12, 13, and 18) and the six lowermost dated samples (from Levels 35–45). The only two outliers are Beta-134576 (Level 19a; possibly younger because of an unlikely intercept of 2,585–2,510 Cal B.P., although the 1σ range is 2,770–2,735 Cal B.P.) and Beta-134581 (Level 33; likely older at 3,220–2,890 Cal B.P., although this time span overlaps that of 10 of the 19 other AMS 14C dates at 2σ). The oldest intercept is from 2,970–2,770 Cal B.P. in every date except Beta-134581.
Table 1.
AMS radiocarbon (14C) ages on individual bones of chicken (Gallus gallus), extinct megapode (Megapodius alimentum), and extinct Tongan iguana (Brachylophus undescribed sp.) from excavation unit 10, Tongoleleka (site Li7), Lifuka Island, Tonga
| Laboratory no. | Species | Skeletal element | Stratum | Level | Depth, cm below datum | Uncorrected 14C age | 13C/12C | Corrected 14C age | Calibrated 14C age |
|---|---|---|---|---|---|---|---|---|---|
| Beta-134573 | G. gallus | Humerus | IIA | 5 | 25/30–33/35 | 2,600 ± 50 | −17.0 | 2,730 ± 50 | 2,935–2,755 |
| Beta-134574 | G. gallus | Coracoid | IIA | 8 | 44/47–52/53 | 2,550 ± 40 | −17.4 | 2,670 ± 40 | 2,845–2,745 |
| Beta-134575 | G. gallus | Tarsometatarsus | IIB | 13 | 70/75–76/79 | 2,540 ± 40 | −18.9 | 2,640 ± 40 | 2,790–2,735 |
| Beta-134576 | G. gallus | Pedal Phalanx | IIC | 19a | 99/100–102/122 | 2,510 ± 60 | −18.7 | 2,610 ± 60 | 2,795–2,710 2,585–2,510 |
| Beta-134577 | G. gallus | Sternum | III | 22 | 106/108–111/113 | 2,450 ± 40 | −16.3 | 2,590 ± 40 | 2,770–2,720 |
| Beta-135249 | G. gallus | Carpometacarpus | III | * | 120–130 | 2,500 ± 40 | −14.9 | 2,670 ± 40 | 2,845–2,745 |
| Beta-134580 | M. alimentum | Carpometacarpus | IIC | 19b | 118–124 (Fea. 2) | 2,710 ± 40 | −20.1 | 2,790 ± 40 | 2,970–2,785 |
| Beta-135250 | M. alimentum | Pedal Phalanx | III | * | 140–160 | 2,670 ± 40 | −20.3 | 2,750 ± 40 | 2,935–2,770 |
| Beta-134581 | M. alimentum | Pedal Phalanx | III/IV | 33 | 145/147–148/151 | 2,830 ± 50 | −19.6 | 2,910 ± 50 | 3,220–2,890 |
| Beta-134582 | M. alimentum | Scapula | III/IV | 34 | 146/148–152/159 | 2,690 ± 40 | −20.2 | 2,770 ± 40 | 2,950–2,775 |
| Beta-134583 | M. alimentum | Pedal Phalanx | III/IV | 35 | 152/159–165/169 | 2,680 ± 50 | −19.4 | 2,770 ± 50 | 2,970–2,770 |
| Beta-134586 | M. alimentum | Humerus | III/IV | 40 | 178–193 (Fea. 5) | 2,660 ± 50 | −19.8 | 2,740 ± 50 | 2,945–2,760 |
| Beta-134584 | M. alimentum | Tarsometatarsus | III/IV | 42 | 186/190–197/199 (Fea. 5) | 2,700 ± 40 | −20.4 | 2,780 ± 40 | 2,960–2,780 |
| Beta-134585 | M. alimentum | Ulna | III/IV | 45 | 202 (Fea. 5) | 2,680 ± 50 | −19.9 | 2,760 ± 50 | 2,960–2,765 |
| Beta-134587 | Brachylophus undescribed sp. | Pedal Phalanx | IIB | 12 | 67/68–70–75 | 2,690 ± 40 | −19.5 | 2,780 ± 40 | 2,960–2,780 |
| Beta-134588 | Brachylophus undescribed sp. | Femur | IIC | 18 | 93/97–95/99 | 2,580 ± 40 | −21.9 | 2,630 ± 40 | 2,785–2,735 |
| Beta-134589 | Brachylophus undescribed sp. | Femur | III | 23 | 111/113–116/119 | 2,560 ± 40 | −19.0 | 2,660 ± 40 | 2,835–2,740 |
| Beta-134590 | Brachylophus undescribed sp. | Thoracic Vertebra | III | 27 | 128/131–132/136 | 2,670 ± 40 | −21.0 | 2,730 ± 40 | 2,890–2,760 |
| Beta-134591 | Brachylophus undescribed sp. | Thoracic Vertebra | III/IV | 31 | 141/144–146/149 | 2,660 ± 40 | −22.8 | 2,700 ± 40 | 2,865–2,755 |
| Beta-134592 | Brachylophus undescribed sp. | Thoracic Vertebra | III/IV | 34 | 146/148–152/159 | 2,640 ± 50 | −22.1 | 2,680 ± 50 | 2,865–2,740 |
Stratum reflects the natural sedimentary structure of the site. Levels are arbitrary within strata, continuous from top to bottom, and average 5 cm in thickness, except for the two samples (Beta-135249, Beta-135250) from adjoining excavation unit 11, where the levels (*) do not correspond to those from unit 10; the stratum and depth of these two samples, however, correlate with those from unit 10. Fea. = Feature. The uncorrected and corrected 14C ages are reported in radiocarbon years before present (yr B.P., with AD 1950 being present). The calibrated 14C ages (95% confidence interval) are calibrated calendrically for secular variation in atmospheric 14C, reported as Cal B.P. following ref. 26.
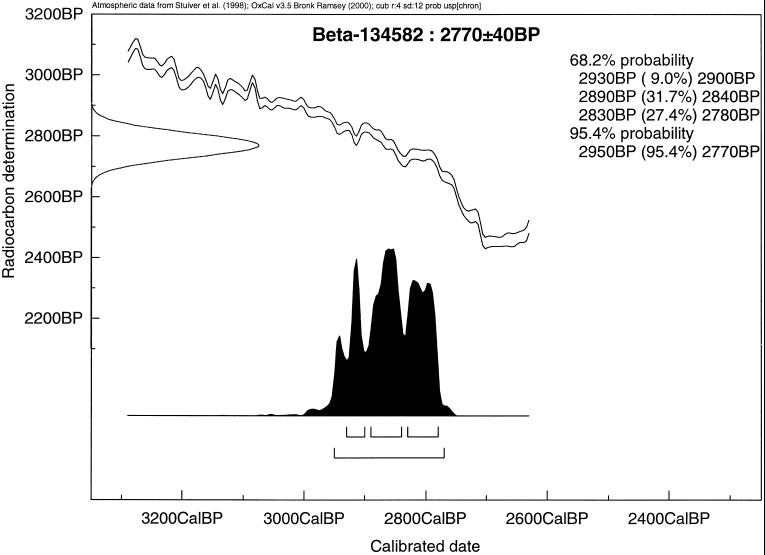
The slope of the radiocarbon calibration curve varies considerably through the Holocene (26). In plotting uncalibrated 14C dates versus those that are calendrically calibrated by tree-ring absolute chronology, an extremely steep slope could mean that uncalibrated 14C dates that differ by centuries would yield similar calibrated dates. Conversely, a more or less flat curve would make the uncalibrated 14C dates yield wide-ranging, broadly overlapping calibrated dates, thereby providing poor chronologic resolution. Fortunately, for the period of interst (≈3,000–2,700 Cal B.P.), the slope of the calibration curve is not particularly flat or steep (i.e., Fig. 4). Thus, the high overall similarity in our calibrated dates is not an artifact of the 14C calibration curve.
Figure 4.
Calibration curve for Beta-134582, an AMS 14C date based on a scapula of Megapodius alimentum from Layer III/IV, level 34, Unit 10, Tongoleleka (site Li7), Lifuka, Tonga.
Interpreted stratigraphically (Table 2), the weighted mean averages of our AMS 14C dates overlap broadly from Layer IIA (2,850–2,760 Cal B.P.) through Layer III/IV (2,880–2,790 Cal B.P.), thus suggesting that the entire cultural/faunal sequence at Tongoleleka was deposited during a time interval too brief for chronometric discrimination. When the AMS 14C dates are categorized taxonomically, they average slightly younger for the chicken bones, slightly older for the megapode bones, and intermediate for the iguana bones (Table 2). These subtle differences evaporate when the oldest and youngest dates (Beta-134576 and Beta-134581, respectively) are excluded, but nevertheless conform to the overall vertical distribution of the three species through the stratigraphic profile (Fig. 3).
Table 2.
Stratigraphic and taxonomic clusters of weighted mean averages (rounded to nearest 101) of AMS 14C dates from excavation units 10 and 11, Tongoleleka (site Li7), Lifuka Island, Tonga
| Stratum/species | No. of AMS14C dates | Weighted mean average conventional 14C age, yr B.P. | Weighted mean average calibrated 14C age, Cal B.P. |
|---|---|---|---|
| IIA | 2 | 2,690 ± 30 | 2,850–2,760 |
| IIB/IIC | 5 | 2,700 ± 20 | 2,840–2,760 |
| III | 5 | 2,680 ± 20 | 2,850–2,750 |
| III/IV | 8 | 2,760 ± 20 | 2,880–2,790 |
| Gallus gallus | 6 | 2,650 ± 20 | 2,780–2,750 |
| Megapodius alimentum | 8 | 2,780 ± 20 | 2,950–2,780 |
| Brachylophus undescribed sp. | 6 | 2,700 ± 20 | 2,840–2,760 |
The calibrated 14C ages are presented at 95% C.I. Based on data in Table 1. yr B.P., radiocarbon years before present.
Discussion
Based on weighted mean averages of AMS 14C dates at the Tongoleleka site on Lifuka, Layers IIA, IIB/IIC, and III are essentially the same age as the underlying Layer III/IV. Within this chronometrically unresolvable period, however, major changes took place on Lifuka and other islands in Ha’apai in ceramic style (Lapita to Polynesian Plainware; refs. 12 and 13) and in faunal composition, namely the introduction of chicken, rat, dog, and pig, and the extinction or local extirpation of an iguana, various seabirds, such as shearwaters (Puffinus pacificus, Puffinus lherminieri), a petrel (Pterodroma sp.), prion (Pachyptila sp.), booby (Sula sula), and terns (Sterna anaethetus, Anous stolidus, Anous minutus), and at least 16 species of landbirds including a heron (Nycticorax undescribed sp.), osprey (Pandion haliaetus), megapodes (Megapodius pritchardii, M. alimentum, Megapodius molistructor), rails (cf. Nesoclopeus undescribed sp., Porzana tabuensis), pigeons and doves (Ducula david, Ducula latrans, Didunculus undescribed sp., undescribed genus, Gallicolumba stairi), parrots (Eclectus undescribed sp., Vini solitarius), whistler (Pachycephala sp.), and shrikebill (Clytorhynchus vitiensis) (ref. 25 and D.W.S., unpublished data). Only 10 species of landbirds survive on Lifuka today (27); thus its modern landbird fauna has only about 40% of the species that existed at first human contact. Gone are all species that were flightless and/or believed to be forest obligates.
The pace of prehistoric anthropogenic extinction of birds on oceanic islands was highly variable. After first human contact in the Bismarck Archipelago ≈33,000 years ago (28), the loss of at least 12 species of landbirds on large, rugged, malarial island of New Ireland (7,174 km2, elevation 2,340 m) was protracted over tens of millennia (29). On Mangaia (rugged topography but nonmalarial and much smaller at 52 km2, elevation 169 m) in the remote Cook Islands, palynological evidence from lacustrine sediments argues for first human arrival at ≈2,500 Cal B.P., although archaeological evidence from human habitation sites dates no earlier than ≈1,000 Cal B.P. (30). Regardless of when people first inhabited Mangaia, many of the 11 species of seabirds and 15 species of landbirds lost from this island survived until ≈500 Cal B.P. (refs. 5, 31, and 32, and D.W.S., unpublished data) and therefore coexisted with people for 500–2,000 years. Nonmalarial Lifuka is smaller (11.4 km2), lower (elevation, 16 m), and lacks the topographic features (cliffs, steep mountains, pinnacle karst) that would retard deforestation, agriculture, hunting, and therefore the rate of extinction. It seems logical that a major episode of extinction on Lifuka would be geologically instantaneous.
Subsequent to first human arrival, the timing of late Quaternary extinctions of large vertebrates was also highly variable on continents, ranging from being spread over tens of millennia in Eurasia (33) to a millennium or perhaps even less for some species in Australia and the Americas (34–36). Compared with islands, however, the archaeology of continental extinction is typically elusive, with stratigraphic associations of cultural materials and the remains of extinct species being rare under most circumstances (36).
The rapid and unreversed artifactual and faunal changes that we discovered on Lifuka, from Lapita-style pottery to Polynesian Plainware and from extinct to extant species of vertebrates, are not unique to Lifuka, but occur on each of the Tongan islands where we have developed an extensive archaeological record. Major changes in artifactual and faunal assemblages also characterize the Clovis-to-Folsom transition in North America, distinguishable as well only by statistical averaging of numerous similar 14C dates (38). Just as with the Lapita-to-Polynesian Plainware transition in Tonga, the Clovis peoples in North America are associated with extinct species, whereas the slightly later Folsom peoples postdate faunal collapse. Whether on continents or islands, late Quaternary mass extinction events have left all regions of the earth except Africa with modern vertebrate faunas that have no analog in the fossil record. On a global geographical scale, our permanently impoverished vertebrate communities reflect the late Quaternary dispersal of humans more than any other single factor. On a geological or evolutionary time scale, it is unimportant whether the extinctions required centuries or millennia of human presence.
Acknowledgments
We thank A. Barton, S. Chiu, M. J. Reetz, J. K. Sailer, A. Van Doorn, V. Vi, and M. I. Williams for field or laboratory assistance. Comments by B. J. MacFadden, P. S. Martin, and two anonymous reviewers improved the manuscript. Support was provided by National Science Foundation Grant EAR-9714819 (to D.W.S.), University of Florida Division of Sponsored Research Grant RDA 1-23 95-96 (to D.W.S.), and a Social Science and Humanities Research Council of Canada Grant (to D.V.B.).
Abbreviations
- AMS
accelerator-mass spectrometer
- Cal
calendar years
References
- 1.Diamond J M. Int Counc Bird Preserv Tech Pub. 1985;3:17–21. [Google Scholar]
- 2.James H F, Olson S L. Ornithol Monogr. 1991;46:1–88. [Google Scholar]
- 3.Olson S L, James H F. Ornithol Monogr. 1991;45:1–88. [Google Scholar]
- 4.Steadman D W. Proc Natl Acad Sci USA. 1993;90:818–822. doi: 10.1073/pnas.90.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steadman D W. Science. 1995;267:1123–1131. doi: 10.1126/science.267.5201.1123. [DOI] [PubMed] [Google Scholar]
- 6.Pregill G K, Dye T. Copeia. 1989;1989:505–508. [Google Scholar]
- 7.Pregill G K. Pacific Sci. 1994;47:101–114. [Google Scholar]
- 8.Pregill G K. Copeia. 1998;1998:64–75. [Google Scholar]
- 9.Martin P S. Palaeogeog Palaeoclim Palaeoecol. 1990;82:187–201. [Google Scholar]
- 10.Alroy J. Science. 2001;292:1893–1896. doi: 10.1126/science.1059342. [DOI] [PubMed] [Google Scholar]
- 11.Dye T. Radiocarbon. 1990;32:143–147. [Google Scholar]
- 12.Burley D V. J Polynes Soc. 1994;103:379–411. [Google Scholar]
- 13.Burley D V. In: The Pacific from 5000 to 2000 BP: Colonisation and Transformations. Galipaud J-C, Lilley I, editors. Paris: Éd. IRD; 1999. pp. 189–200. [Google Scholar]
- 14.Dickinson W R, Burley D V, Shutler R., Jr Geoarchaeology. 1994;9:85–111. [Google Scholar]
- 15.Burley D V, Nelson E, Shutler R., Jr Archaeol Oceania. 1995;30:132–134. [Google Scholar]
- 16.Kirch P V. The Lapita Peoples: Ancestors of the Oceanic World. Cambridge, MA: Blackwell; 1997. [Google Scholar]
- 17.Kirch P V. On the Roads of the Winds. Berkeley: Univ. California Press; 2000. [Google Scholar]
- 18.Dickinson W R, Shutler R., Jr J World Prehist. 2000;14:203–266. [Google Scholar]
- 19.Spriggs M. The Island Melanesians. Oxford, U.K.: Blackwell; 1997. [Google Scholar]
- 20.Stafford T W, Jr, Hare P E, Currie L, Jull A J T, Donahue D J. J Archaeol Sci. 1991;18:35–72. [Google Scholar]
- 21.Stafford T W, Jr, Semken H A, Jr, Graham R W, Klippel W F, Markova A K, Smirnov N G, Southon J. Geology. 1999;27:903–906. [Google Scholar]
- 22.Steadman D W, Stafford T W, Jr, Donahue D J, Jull A J T. Quat Res. 1991;35:126–133. [Google Scholar]
- 23.Steadman D W. Proc Biol Soc Wash. 1989;102:537–552. [Google Scholar]
- 24.Steadman D W. Zool Verhand. 1999;327:7–21. [Google Scholar]
- 25. Steadman, D. W., Plourde, A. M. & Burley, D. V. (2002) J. Archaeol. Sci, in press.
- 26.Stuiver M, Reimer P J, Bard E, Beck W E, Burr G S, Hughen K A, Kromer B, McCormac F V, van der Plicht J, Spurk M. Radiocarbon. 1998;40:1041–1083. [Google Scholar]
- 27.Steadman D W. Pacific Sci. 1998;52:14–34. [Google Scholar]
- 28.Leavesley M, Allen J. Archaeol Oceania. 1998;33:63–82. [Google Scholar]
- 29.Steadman D W, White J P, Allen J. Proc Natl Acad Sci USA. 1999;96:2563–2568. doi: 10.1073/pnas.96.5.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirch P V, Ellison J. Antiquity. 1994;68:310–321. [Google Scholar]
- 31.Kirch P V, Steadman D W, Butler V L, Hather J, Weisler M I. Archaeol Oceania. 1995;30:47–65. [Google Scholar]
- 32.Steadman D W, Antón S C, Kirch P V. Antiquity. 2000;74:873–883. [Google Scholar]
- 33.Stuart A J. Biol Rev. 1991;66:453–562. doi: 10.1111/j.1469-185x.1991.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 34.Alroy J. In: Extinctions in Near Time. MacPhee R D E, editor. New York: Kluwer Academic/Plenum; 1999. pp. 105–143. [Google Scholar]
- 35.Martin P S, Steadman D W. In: Extinctions in Near Time. MacPhee R D E, editor. New York: Kluwer Academic/Plenum; 1999. pp. 17–55. [Google Scholar]
- 36.Roberts R G, Flannery T F, Ayliffe L K, Yoshida H, Olley J M, Prideaux G J, Laslett G M, Baynes A, Smith M A, Jones R, Smith B L. Science. 2001;292:1888–1892. doi: 10.1126/science.1060264. [DOI] [PubMed] [Google Scholar]
- 37.Haynes G, Eiselt B S. In: Extinctions in Near Time. MacPhee R D E, editor. New York: Kluwer Academic/Plenum; 1999. pp. 71–93. [Google Scholar]
- 38.Taylor R E, Haynes C V, Jr, Stuiver M. Antiquity. 1996;70:515–525. [Google Scholar]