Abstract
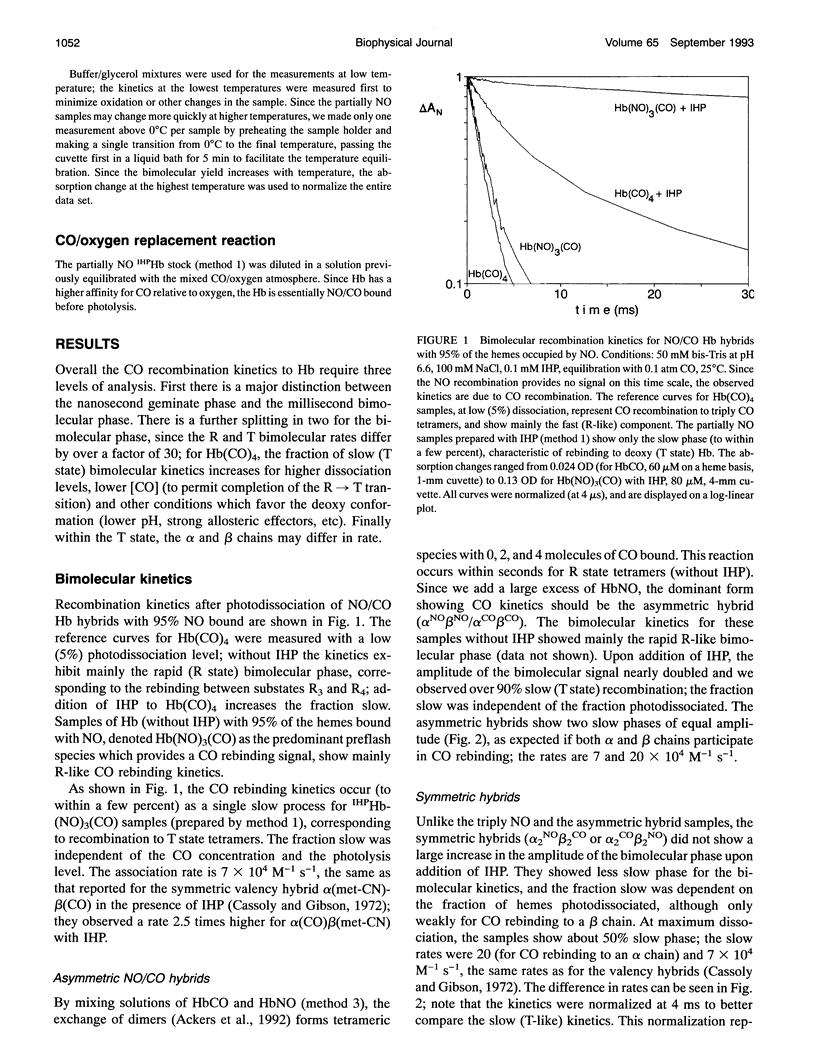
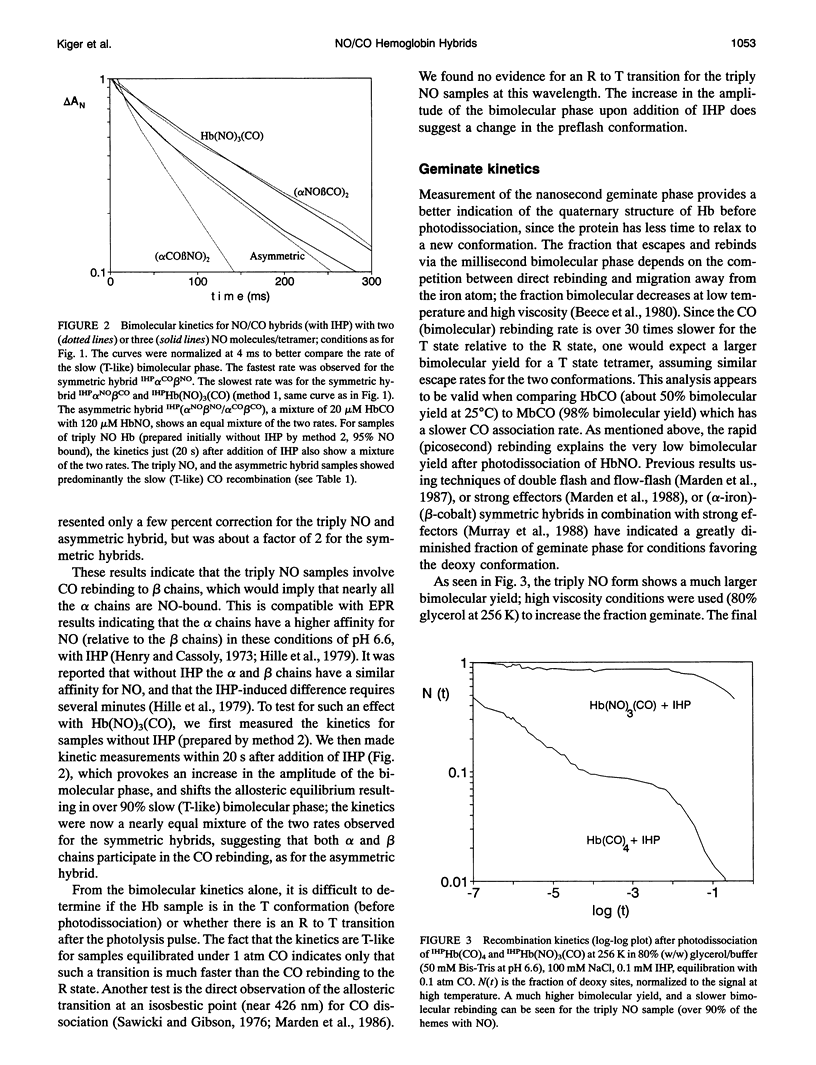
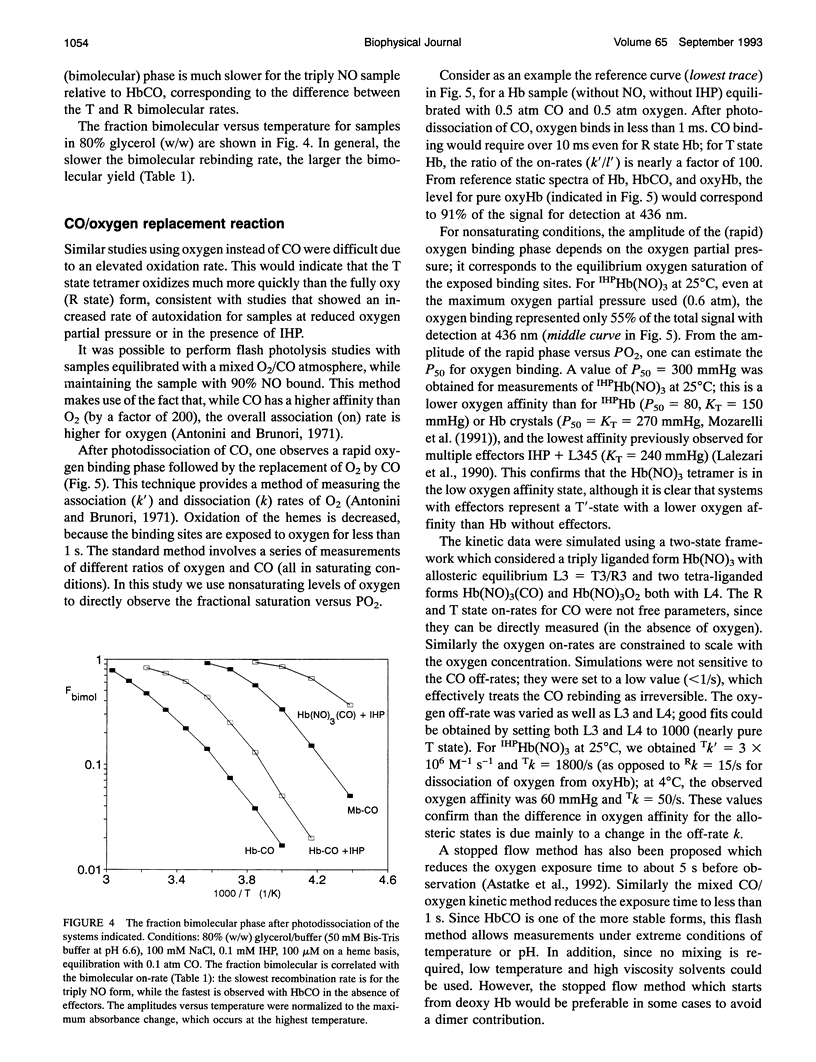
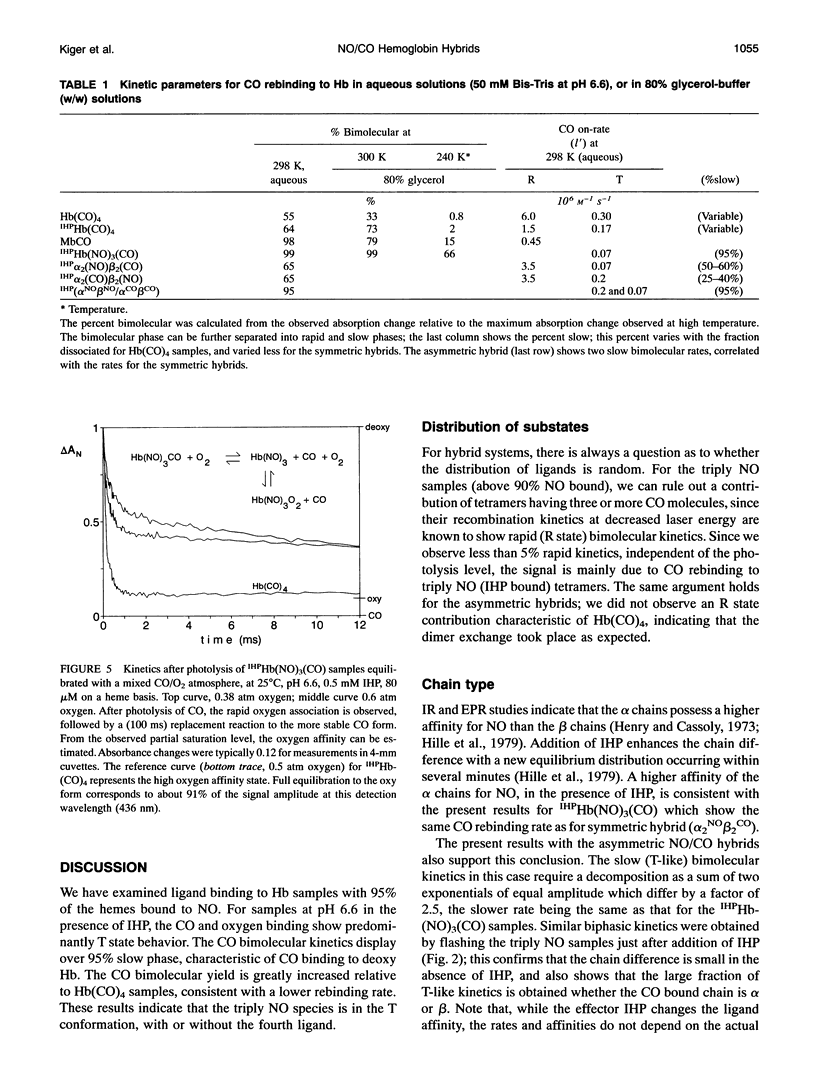
The bimolecular and geminate CO recombination kinetics have been measured for hemoglobin (Hb) with over 90% of the ligand binding sites occupied by NO. Since Hb(NO)4 with inositol hexaphosphate (IHP) at pH below 7 is thought to take on the low affinity (deoxy) conformation, the goal of the experiments was to determine whether the species IHPHb-(NO)3(CO) also exists in this quaternary structure, which would allow ligand binding studies to tetramers in the deoxy conformation. For samples at pH 6.6 in the presence of IHP, the bimolecular kinetics show only a slow phase with rate 7 x 10(4) M-1 s-1, characteristic of CO binding to deoxy Hb, indicating that the triply NO tetramers are in the deoxy conformation. Unlike Hb(CO)4, the fraction recombination occurring during the geminate phase is low (< 1%) in aqueous solutions, suggesting that the IHPHb(NO)3(CO) hybrid is also essentially in the deoxy conformation. By mixing stock solutions of HbCO and HbNO, the initial exchange of dimers produces asymmetric (alpha NO beta NO/alpha CO beta CO) hybrids. At low pH in the presence of IHP, this hybrid also displays a high bimolecular quantum yield and a large fraction of slow (deoxy-like) CO recombination; the slow bimolecular kinetics show components of equal amplitude with rates 7 and 20 x 10(4) M-1 s-1, probably reflecting the differences in the alpha and beta chains. Samples of symmetric hybrids (a2NOI32Co or a2Co922NO) showed a lower (R-like) bimolecular yield and less slow phase for the CO bimolecular recombination, relative to the asymmetric hybrid or the triply NO species. The slower (T state) bimolecular rate of 7 x 104 M-1 s-1 was observed for CO rebinding to a chain.While oxygen equilibrium studies with 'HPHb(NO)3 were hampered by a high oxidation rate, it was possible to perform experiments with samples equilibrated with a mixed CO/oxygen atmosphere. Photodissociation of CO allows a temporary exposure of the binding sites to oxygen. The results confirm that IHPHb(NO)3 has a low oxygen affinity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astatke M., McGee W. A., Parkhurst L. J. A flow procedure to determine oxygen binding isotherms for low affinity and easily oxidized hemoglobins. Comp Biochem Physiol B. 1992 Apr;101(4):683–688. doi: 10.1016/0305-0491(92)90359-y. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay D., Magde D., Traylor T. G., Sharma V. S. Quaternary structure and geminate recombination in hemoglobin: flow-flash studies on alpha 2CO beta 2 and alpha 2 beta 2CO. Biophys J. 1992 Sep;63(3):673–681. doi: 10.1016/S0006-3495(92)81652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beece D., Eisenstein L., Frauenfelder H., Good D., Marden M. C., Reinisch L., Reynolds A. H., Sorensen L. B., Yue K. T. Solvent viscosity and protein dynamics. Biochemistry. 1980 Nov 11;19(23):5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- Bonaventura J., Riggs A. Hemoglobin Kansas, a human hemoglobin with a neutral amino acid substitution and an abnormal oxygen equilibrium. J Biol Chem. 1968 Mar 10;243(5):980–991. [PubMed] [Google Scholar]
- Briehl R. W., Salhany J. M. Gelation of sickle hemoglobin. III. Nitrosyl hemoglobin. J Mol Biol. 1975 Aug 25;96(4):733–743. doi: 10.1016/0022-2836(75)90149-7. [DOI] [PubMed] [Google Scholar]
- Brittain T. The Root effect. Comp Biochem Physiol B. 1987;86(3):473–481. doi: 10.1016/0305-0491(87)90434-2. [DOI] [PubMed] [Google Scholar]
- Cassoly R., Gibson Q. H. The kinetics of ligand binding to hemoglobin valency hybrids and the effect of anions. J Biol Chem. 1972 Nov 25;247(22):7332–7341. [PubMed] [Google Scholar]
- Cassoly R. Relation entre spectre d'absorption optique et structure de la nitrosyl hémoglobine. C R Acad Sci Hebd Seances Acad Sci D. 1974 Mar 4;278(10):1417–1420. [PubMed] [Google Scholar]
- Cassoly R. Relations between optical spectrum and structure in nitrosyl hemoglobin and hybrids. J Mol Biol. 1975 Nov 5;98(3):581–595. doi: 10.1016/s0022-2836(75)80088-x. [DOI] [PubMed] [Google Scholar]
- Cassoly R. Use of nitric oxide as a probe for assessing the formation of asymmetrical hemoglobin hybrids. An attempted comparison between alphaNObetaNOalphadeoxybetadeoxy, alpha2NObeta2deoxy, and alpha2deoxybeta2NO hybrids. J Biol Chem. 1978 May 25;253(10):3602–3606. [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. M., Scott T. W., Stepnoski R. A., Ikeda-Saito M., Yonetani T. The iron-proximal histidine linkage and protein control of oxygen binding in hemoglobin. A transient Raman study. J Biol Chem. 1983 Sep 10;258(17):10564–10572. [PubMed] [Google Scholar]
- GIBSON Q. H. The direct determination of the velocity constant of the reaction Hb4 (CO)3 + CO-Hb4(CO)4. J Physiol. 1956 Oct 29;134(1):123–134. doi: 10.1113/jphysiol.1956.sp005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. D., Gibson Q. H. The effect of inositol hexaphosphate on the kinetics of CO and O 2 binding by human hemoglobin. J Biol Chem. 1971 Dec 10;246(23):7168–7174. [PubMed] [Google Scholar]
- Henry Y., Cassoly R. Chain non-equivalence in nitric oxide binding to hemoglobin. Biochem Biophys Res Commun. 1973 Apr 2;51(3):659–665. doi: 10.1016/0006-291x(73)91365-x. [DOI] [PubMed] [Google Scholar]
- Hille R., Olson J. S., Palmer G. Spectral transitions of nitrosyl hemes during ligand binding to hemoglobin. J Biol Chem. 1979 Dec 10;254(23):12110–12120. [PubMed] [Google Scholar]
- Ignarro L. J. Heme-dependent activation of soluble guanylate cyclase by nitric oxide: regulation of enzyme activity by porphyrins and metalloporphyrins. Semin Hematol. 1989 Jan;26(1):63–76. [PubMed] [Google Scholar]
- Kosaka H., Uozumi M., Tyuma I. The interaction between nitrogen oxides and hemoglobin and endothelium-derived relaxing factor. Free Radic Biol Med. 1989;7(6):653–658. doi: 10.1016/0891-5849(89)90146-9. [DOI] [PubMed] [Google Scholar]
- Lalezari I., Lalezari P., Poyart C., Marden M., Kister J., Bohn B., Fermi G., Perutz M. F. New effectors of human hemoglobin: structure and function. Biochemistry. 1990 Feb 13;29(6):1515–1523. doi: 10.1021/bi00458a024. [DOI] [PubMed] [Google Scholar]
- Marden M. C. A coupled diffusion and barrier model for the recombination kinetics of myoglobin with carbon monoxide. Eur J Biochem. 1982 Nov 15;128(2-3):399–404. doi: 10.1111/j.1432-1033.1982.tb06978.x. [DOI] [PubMed] [Google Scholar]
- Marden M. C., Hazard E. S., Gibson Q. H. Testing the two-state model: anomalous effector binding to human hemoglobin. Biochemistry. 1986 Nov 18;25(23):7591–7596. doi: 10.1021/bi00371a049. [DOI] [PubMed] [Google Scholar]
- Marden M. C., Hazard E. S., Kimble C., Gibson Q. H. Geminate ligand recombination as a probe of the R, T equilibrium in hemoglobin. Eur J Biochem. 1987 Dec 15;169(3):611–615. doi: 10.1111/j.1432-1033.1987.tb13652.x. [DOI] [PubMed] [Google Scholar]
- Marden M. C., Kister J., Bohn B., Poyart C. T-state hemoglobin with four ligands bound. Biochemistry. 1988 Mar 8;27(5):1659–1664. doi: 10.1021/bi00405a041. [DOI] [PubMed] [Google Scholar]
- Maxwell J. C., Caughey W. S. An infrared study of NO bonding to heme B and hemoglobin A. Evidence for inositol hexaphosphate induced cleavage of proximal histidine to iron bonds. Biochemistry. 1976 Jan 27;15(2):388–396. doi: 10.1021/bi00647a023. [DOI] [PubMed] [Google Scholar]
- Moore E. G., Gibson Q. H. Cooperativity in the dissociation of nitric oxide from hemoglobin. J Biol Chem. 1976 May 10;251(9):2788–2794. [PubMed] [Google Scholar]
- Mozzarelli A., Rivetti C., Rossi G. L., Henry E. R., Eaton W. A. Crystals of haemoglobin with the T quaternary structure bind oxygen noncooperatively with no Bohr effect. Nature. 1991 May 30;351(6325):416–419. doi: 10.1038/351416a0. [DOI] [PubMed] [Google Scholar]
- Murray L. P., Hofrichter J., Henry E. R., Ikeda-Saito M., Kitagishi K., Yonetani T., Eaton W. A. The effect of quaternary structure on the kinetics of conformational changes and nanosecond geminate rebinding of carbon monoxide to hemoglobin. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2151–2155. doi: 10.1073/pnas.85.7.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Hori H., Yoshida S., Sakamoto H., Morimoto H. The effect of quaternary structure on the state of the alpha and beta subunits within nitrosyl haemoglobin. Low temperature photodissociation and the ESR spectra. Biochim Biophys Acta. 1978 Jan 25;532(1):17–28. doi: 10.1016/0005-2795(78)90443-9. [DOI] [PubMed] [Google Scholar]
- Perrella M., Colosimo A., Benazzi L., Ripamonti M., Rossi-Bernardi L. What the intermediate compounds in ligand binding to hemoglobin tell about the mechanism of cooperativity. Biophys Chem. 1990 Aug 31;37(1-3):211–223. doi: 10.1016/0301-4622(90)88020-s. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Kilmartin J. V., Nagai K., Szabo A., Simon S. R. Influence of globin structures on the state of the heme. Ferrous low spin derivatives. Biochemistry. 1976 Jan 27;15(2):378–387. doi: 10.1021/bi00647a022. [DOI] [PubMed] [Google Scholar]
- Saffran W. A., Gibson Q. H. Photodissociation of ligands from heme and heme proteins. Effect of temperature and organic phosphate. J Biol Chem. 1977 Nov 25;252(22):7955–7958. [PubMed] [Google Scholar]
- Salhany J. M., Ogawa S., Shulman R. G. Spectral-kinetic heterogeneity in reactions of nitrosyl hemoglobin. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3359–3362. doi: 10.1073/pnas.71.9.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches R. Dehydration effects on the heme environment of nitric oxide hemoglobin. Biochim Biophys Acta. 1988 Aug 10;955(3):310–314. doi: 10.1016/0167-4838(88)90209-9. [DOI] [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Properties of the T state of human oxyhemoglobin studies by laser photolysis. J Biol Chem. 1977 Nov 10;252(21):7538–7547. [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Quaternary conformational changes in human hemoglobin studied by laser photolysis of carboxyhemoglobin. J Biol Chem. 1976 Mar 25;251(6):1533–1542. [PubMed] [Google Scholar]
- Sharma V. S., Ranney H. M. The dissociation of NO from nitrosylhemoglobin. J Biol Chem. 1978 Sep 25;253(18):6467–6472. [PubMed] [Google Scholar]
- Shulman R. G., Hopfield J. J., Ogawa S. Allosteric interpretation of haemoglobin properties. Q Rev Biophys. 1975 Jul;8(3):325–420. doi: 10.1017/s0033583500001840. [DOI] [PubMed] [Google Scholar]
- Szabo A. Kinetics of hemoglobin and transition state theory. Proc Natl Acad Sci U S A. 1978 May;75(5):2108–2111. doi: 10.1073/pnas.75.5.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traylor T. G., Sharma V. S. Why NO? Biochemistry. 1992 Mar 24;31(11):2847–2849. doi: 10.1021/bi00126a001. [DOI] [PubMed] [Google Scholar]
- Wajnberg E., Linhares M. P., el-Jaick L. J., Bemski G. Nitrosyl hemoglobin: EPR components at low temperatures. Eur Biophys J. 1992;21(1):57–61. doi: 10.1007/BF00195444. [DOI] [PubMed] [Google Scholar]
- Zijlstra W. G., Buursma A., Meeuwsen-van der Roest W. P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin Chem. 1991 Sep;37(9):1633–1638. [PubMed] [Google Scholar]


